You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Sleep disordered breathing (SDB) encompasses a spectrum of dysfunctional sleep breathing, including occasional snoring, habitual snoring, upper airway resistance syndrome (UARS), sleep apnea, and hypoventilation. The medical community has traditionally focused on obstructive sleep apnea (OSA) and obesity hypoventilation syndrome. Unfortunately, many sleep-related breathing disorders, especially those predominately found in women and children, have been ignored because of the focus on OSA.
Snoring typically occurs when air passes between the tongue and soft palate, causing a vibration of the soft palate. A snoring sound may also be produced from the nose during inhalation. Children can produce the same loud snoring sound as an adult, but typically their snoring is more of an effortful breathing, making recognition and diagnosis more challenging in this population.
The consequences of snoring can be serious. Habitual snoring, defined as three times per week or more, has been associated with hyperactive behavior in children as young as 3 years of age1 and poor academic performance.2 Sleep fragmentation or disruption caused by snoring appears to play a role as important as hypoxia in causing dysfunction. Benign snoring in adults has been implicated in an increase risk of stroke.3
UARS and OSA: A Comparison
Although many clinicians describe UARS and OSA as the same disease with a slight variance in severity, their pathophysiologies appear to be different.4 OSA is characterized by complete upper airway obstructions lasting longer than 10 seconds with an associated 4% oxygen desaturation. It is most commonly attributed to a hypotonia of the soft palate or base of tongue. Partial airway obstructions that lead to desaturation or brief awakenings from sleep are classified as hypopneas. Continued desaturations over time may cause excessive daytime sleepiness and hypertension. They have been correlated to endothelial dysfunction, myocardial infarction, and cerebrovascular accidents. The level of severity of OSA has been associated with an increased mortality.5,6
Anatomic irregularities or minor breathing impairments can create UARS.7 Patients with UARS may have a more collapsible airway because of abnormal inspiratory flow dynamics8 or increased collapsibility on expiration due to atypical anatomy.9 UARS patients have more sensitivity to restricted breathing or negative oropharyngeal pressure. The airway constriction is recognized and responded to more quickly, preventing obstruction. These respiratory effort–related arousals (RERAs) and sleep fragmentations lead to activation of the autonomic nervous system—in particular, increased sympathetic nerve activity.10
Sleep Dentistry
Continuous positive airway pressure (CPAP) was introduced in 1981.11 CPAP is still the standard of care today for OSA.12 Even with significant improvements in CPAP technology, it is unpopular with patients, rarely worn throughout an entire night,13 and has less than optimal long-term compliance.14
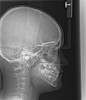
Oral appliances (OA) were introduced in the 1980s in an attempt to provide an alternative to the unpopular CPAP. OAs act by protruding the mandible and attempting to position the tongue out of the oropharyngeal region. OAs are currently divided by their manner of therapy. Tongue-retaining devices utilize negative pressure from a bulb attached to the tip of the tongue to reposition the tongue. Mandibular advancing appliances (MAAs) are attached to the dental arch (Figure 1). The mandible is held in a protruded position. The protrusion is either fixed or titratable.
Although the quest to create an alternative to CPAP is understandable, sleep dentistry has become single-minded in its treatment of adult apnea with an appliance. If dentistry compartmentalizes itself on OA fabrication for OSA, women with UARS and the majority of children are eliminated from the purview of the dental practitioner.
Sleep Prosthodontics
Sleep dentistry can be thought of as the study of an OA and its impact on the airway. Sleep prosthodontics is the study of the airway and its impact on the stomatognathic system. The stomatognathic system encompasses the mouth, jaws, and the closely related structures of the oro-pharynx and fauces. Dentists deal with this system during its development and maintain it throughout a lifetime.
Although a physician must make the diagnosis of SDB, the dentist plays a critical “diagnostic” role. Many times the lack of witnessed apneic episodes or the lack of particularly egregious daytime symptoms may lead to a delay in care by the medical community. The impact of a poor airway can many times be detected in the patient’s craniofacial development, oral impairment, and occlusal dysfunction well before the clinical presentation of systemic disease.15
Sleep prosthodontics is not restricted to an appliance, but instead has a single-minded focus on the patient’s health. It also encourages a patient-centered, interdisciplinary solution that includes a wide range of options, including orthodontics, oral mycology, nutrition/diet counseling, orthognathics, CPAP, MAA, and otolaryngologic surgeries.
The Snoring Child
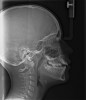
In the general population, 2% to 3% of children have apnea.16 That proportion is growing given the increase in childhood obesity.17 Apneas and hypopneas are defined in children as events lasting longer than two missed breaths and most commonly associated with some change in oxygen saturation or end tidal CO2 increase. UARS presents in children during polysomnography (PSG) as an increased respiratory effort with no apnea and little oxygen saturation change (Figure 2). The characteristic signs and symptoms of UARS vary with the age of the child, as will treatment options.18
The prevalence of snoring in children ranges from 10% to 21% from 6 to 81 months.19 Habitual snoring has been reported in 9% of infants aged 0 to 3 months.20 In a general pediatric clinic, habitual snoring was documented in 17% of patients, with that rising to 29% of the children reporting for neurologic indications such as headaches and 56% of the children diagnosed with psychiatric disorders (half with anxiety/mood disorders).21,22 In a 2-year follow-up on habitually snoring children, 30% of subjects had worsened from baseline. OSA developed more often in boys, especially if adenotonsillar hypertrophy or an increase in waist circumference was present.23
Snoring and mouth breathing in children were initially thought of as unreliable markers for OSA and not as potential problems in their own right. More recently, it is believed that snoring independent of OSA may cause neurocognitive dysfunction and impaired daytime performance.24,25 Habitually snoring children are at higher risk for social problems, poor academic performance, decreased attention, and anxiety/depression issues.26-28 Children who are chronic snorers have abnormal slow-wave sleep patterns and experience more fragmentation. This sleep instability may explain the detrimental effects of non-apneic snoring.29 Studies of occasionally snoring children who otherwise have normal sleep demonstrated altered brain function and more delayed and effortful processing. These children also experienced more behavioral problems than non-snoring children.30 Children who snore are not likely to “grow out of it” without experiencing cognitive impairment.
The neurocognitive and behavioral damage from snoring in children appears to be related to the fact that their brains are still developing. A confounding issue is that the impact of the snoring may not be detected for years, even after the snoring has resolved. The genesis of the long-term neurocognitive effect in snoring children may be during a critical developmental period—at or before 3 years of age.
Bonuck and colleagues31 examined 7 years of epidemiologic data from more than 11,000 children followed from birth. Cognitive and behavioral assessments were conducted when the children reached 4 and 7 years of age. By 4 years old, children who had a history of SDB were 20% to 60% more likely to exhibit behavioral difficulties; by 7 years, they were 40% to 100% more likely. The more severe SDB was linked to the poorest behavioral outcomes. The “Worst Case” cluster had a peak of SDB symptoms at 30 months that abated. Nonetheless, at 7 years the cluster displayed hyperactivity and conduct and peer difficulties. Inclusion in the “Later SDB Symptom” cluster, with a peak at 42 through 69 months, was predictive of emotional difficulties and hyperactivity at both 4 and 7 years.
Bonuck’s work underscores that the presence of irregular sleep breathing may not be directly linked to the academic and behavioral symptoms. Instead, SDB during periods of brain development is very predictive of later damage. The neurocognitive damage in areas such as academic performance and executive functioning is not reversible, so early identification and treatment are paramount.32-34
Metabolic Consequences
Because of the close link between sleep, the immune system, and inflammation, children with SDB are prone to many of the same systemic inflammatory conditions that as adults lead to high blood pressure, arrhythmias, and congestive heart failure.35 Sleep disturbances in children lead to aberrant sympathetic nervous activation that creates cardiovascular and metabolic injury.36 Pediatric apnea is connected with endothelial microvascular dysfunction: a marker of subclinical cardiovascular disease, systemic hypertension, pulmonary hypertension, and myocardial left ventricular remodeling.37 In the presence of obesity, the metabolic consequences are exacerbated. Treatment of the SDB is mandatory to prevent complications.38 If the child’s SDB is resolved, the systemic inflammation in non-obese subjects appears to be reversible.39 The unanswered question is whether the childhood autonomic disturbance promotes metabolic morbidity later in life even after SDB resolution.35
Sleep Prosthodontics and the Snoring Child
The American Academy of Pediatrics (AAP) guidelines for the diagnosis and management of childhood OSA syndrome40 call for every child/adolescent to be screened for snoring at each office visit. A PSG should be ordered on children who snore and have neurocognitive, behavioral, or medical issues indicative of OSA. The goal of the guidelines is to screen for more SDB, but there are two diagnostic problems. Although PSG is the gold standard for diagnosis of OSAs, there is a shortage of sleep laboratories with pediatric expertise and equipment.40 In addition, the worst-case children have no behavioral issues when the snoring is occurring. It is not until years later that the hyperactivity and emotional issues arise, after the snoring has abated.31 Physicians following the AAP guidelines will not discover the worst SDB children. Dentists and dental hygienists have a unique role in early identification of SDB in children. Beginning sleep prosthodontic indicators of SDB are craniofacial anomalies resulting in malocclusions and sleep-related bruxing.
Abnormal Craniofacial Growth
SDB and abnormal craniofacial development are bidirectional. SDB may create craniofacial changes. These skeletal alterations can further exacerbate the SDB difficulty. Treating the breathing issue as early as possible prevents the continuation or the worsening of the craniofacial problems. It may also lead to an improvement in growth and development if addressed soon enough in the process. Before the age of 4 years, 60% of craniofacial maturity is completed. Approximately 90% is finished by the age of 12 years.41 Tonsils and adenoids begin hypertrophying at 2.5 years, reaching their greatest dimensions around 5 or 6 years. Early and late growth clusters exist in children with SDB. The early cluster will have SDB without tissue hypertrophy. The late cluster will react to the additional airway blockage.
Early Growth Cluster
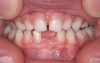
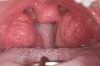
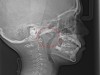
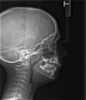
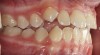
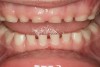
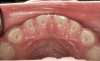
The pattern for bone growth resides not in the bone itself but rather in the soft tissue and muscle that encase the bone.42 Oral-facial muscle tone and tongue tonicity create a framework for normal development of the nasomaxillary complex and mandible.43 SDB is noted in children with pathologic hypotonia of facial and tongue muscles. Children born with a normal palate and oral-facial hypotonia will develop a high, narrow palate over the first year of life (Figure 3 and Figure 4). Children born with a high, narrow palate have hypotonia at birth. These myofunctional changes may be detected in utero.43
Premature children suffer from SDB and OSA due to the lack of completion of craniofacial development in utero. They typically have a narrow, hard palate, abnormal nasal resistance, and mouth breathing. These changes promote the development of an abnormally long lower third of the face.44 Kin and colleagues concluded that if nothing is done in these premature infants, SDB and OSA will develop.44
Instinctively, clinicians have concentrated on the mandible when discussing airway dimensions. However, the maxilla appears to be the more important arch in determining upper airway dimensions in OSA patients.45 The distance from A point (most posterior point in the concavity of the anterior maxilla) to Porion vertical (vertical line drawn from the most superior part of the external auditory meatus) was the most contributory cephalometric marker for airway patency. Appropriate positioning of the maxilla opens the velopharyngeal and orophayngeal airways. Additionally, proper maxillary positioning enhances mandibular growth. Thus, the lack of facial muscle activity and ideal tongue tone constrains the premaxilla, producing an abnormal airway dimension and amplifying the threat of SDB.
Late and Mixed Growth Cluster
Tonsils and adenoids occupy space, increase airway resistance, and create turbulent nasal airflow. The period of tissue hypertrophy is especially damaging to mixed cluster patients. These children are undiagnosed early growth cluster children who become further compromised due to enlargement of oral and nasal tissues.
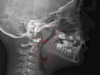
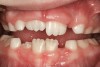
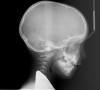
Tonsils and adenoids should be judged against the relative size of the airway rather than the absolute size of the lymphoid tissue46 (Figure 5 and Figure 6). Adenoids are located at the posterior of the nasal cavity on the roof of the nasopharynx (Figure 7). The normal distance from the adenoids to the soft palate for an acceptable airway should be at least 12 mm. For each millimeter decrease, the odds of the child snoring increase 1.61 times. Mouth breathers typically show a smaller upper airway dimension as well.47 The adenoid and tonsillar obstruction creates the trigger, but the deviate facial and neck muscle recruitment and tongue hypotonia cause the maldevelopment.48
The point of obstruction tends to determine the type of skeletal impact. Nasal obstruction from enlarged turbinates, blocked ostium maxillare, deviated septum, or nasal valve stenosis creates Angle occlusions of Class I, II, and III equally (Figure 8). The maxilla in these cases is positioned posteriorly and the mandible is posterior-inferior. The facial type is most commonly dolicocephalic. Blockage of the airway predominately by the adenoids will create growth patterns that yield mostly Class II occlusions and anterior open bite with both jaws located posterior-inferiorly. Facial type is again dolicocephalic with the typical long-thin “adenoidal” face49 (Figure 9 through Figure 12). If the tonsillar tissue is responsible for the airway obstruction, the tongue will have an abnormal resting posture. Class III occlusions will be more common with the maxilla normal or posterior placed (Figure 13 through 15). The tongue may direct the mandible anteriorly or, because the tongue is not in the roof of the mouth driving A point anterior, the maxilla will become bimaxillary retrusive.50 In some cases, the anterior posture of the tongue will create an open bite. This is incorrectly referred to as a tongue thrust. The impact from a thrust does not alter the tooth position. Long-term, low forces cause tooth movement. The posture of the tongue against or between the anterior teeth due to the excessive tonsillar size creates the open bite (Figure 16 and Figure 17). Facial types in this group are more brachyfacial. Lastly, if the airway is blocked through a combination of factors, the Angle classification will be either Class II or III. The maxilla will be in a normal location and the mandible will be the affected arch (Figure 18). These craniofacial changes are not restricted to OSA; all SDB will create unique alterations depending on the patient compensation. Children with UARS have been reported to display high, narrow palates, dolicofacial form, and a Class II malocclusion, indicative of largely adenoidal blockage.51
Dentists identifying craniofacial changes early in development may resolve the malocclusion by simply referring for adenotonsillectomy (T&A). The impact of T&A on the pediatric immune system is controversial. A recent 5-year longitudinal, prospective study demonstrated that adenotonsillectomy does not pose adverse short- or long-term impact on the cellular or humoral immunity.52 Cephalametric changes (eg, posterior incline to the mandible, anterior incline to the maxilla, longer anterior and shorter posterior face height, and upper and lower teeth more retroclined than a normal matched control) were detected in 5-year-old subjects with adenoid-induced OSA.53 T&A resolved the OSA in all subjects. At the 5-year recall, cephalometric evaluation demonstrated that the mandibular plane angle and incisor relationship was similar to the control. Early resolution of the SDB allowed time for the proper use of the oral-facial and tongue muscles. Closed mouth breathing with the tongue in the roof of the mouth directed ideal growth.
In some children, T&A alone may not completely resolve the OSA (Figure 19 and Figure 20). The longer the airway dysfunction, the greater the structural impact on the airway. An interdisciplinary clinical study54 was conducted on children approximately 6.5 years old with inclusion criteria of OSA, large tonsils, visually constricted airway, and high and narrow palate. Group 1 was treated with rapid maxillary expansion (RME) and Group 2 with T&A. Maxillary expansion has been shown to create improved nasal resistance and an increase nasal cavity volume. In cases without excessive lymphoid hypertrophy, RME can resolve significant levels of OSA.55,56 After the original therapy, only one child had been completely resolved (apnea-hypopnea index [AHI] <1). The remaining subjects switched groups and received the opposite treatment. After receiving both treatments, 29 of the 31 children were cured. It can be concluded that many children must be treated with multiple therapies before resolution, especially if the SDB has previously altered the airway to a significant degree.
Sleep Bruxism
Not only are dentists in the best position to detect and intercede in cases of abnormal craniofacial development, but they are also the best judges of aberrant tooth wear. Bruxism occurs in up to 30% of children, often around 5 and 6 years during late cluster adenoid and tonsillar hypertrophy.57 Carlsson and colleagues58 determined in a 20-year prospective study that bruxism in childhood may be a persistent trait. Early tooth wear was predictive of increased tooth wear 20 years later. The results emphasize that the triggering mechanism for sleep bruxism is present as a child and does not develop over time.
For restorative dentists, it is significantly more important to locate what elicits the action than the “genetic code” that produces a bruxer. Historically, popular theories have postulated that the generator for bruxism was stress, neurochemical, or occlusion. PSG-based research has disproven these theories. Stress leads to awake bruxism, not sleep bruxism.59,60 Most chemical irregularities in bruxers are linked to sleep fragmentation.61,62 Finally, bruxism is a centrally, not peripherally, mediated event.63 Idealizing occlusions may control the impact of bruxism and improve chewing function, but it will not resolve sleep bruxism.64
Bruxism occurs during microarousals from regular sleep patterns.64,65 Many factors may introduce microarousals, including reflux and tactile and auditory stimuli.66,67 The most common reason for these bruxism-related microarousals appears to be respiratory effort. It is the author’s assertion that sleep bruxism serves a functional role in protecting and improving the airway during episodes of inspiratory flow limitation and obstruction. The activity of increasing genioglossal and infrahyoid muscle tone along with the lateral movement of the mandible dilates the upper airway, raises inspiratory flow, and reduces upper airway resistance.68
Sleep bruxism is classified as a sleep-related movement disorder similar to restless leg syndrome and is routinely referred to as a possible indicator of SDB. The majority of bruxism occurs during light non–rapid eye movement sleep.69 With traditional PSG, 80% or more of the bruxing episodes have related respiratory events.68,70 Linking bruxism and airway resistance causally is difficult, given that many abnormal breathing patterns are not necessarily conspicuous on PSG. Sleep apneas are more easily recognized, but the RERAs can be a challenge to identify, especially in children who do not desaturate like adults. Additionally, bruxism minimizes the degree of obstruction and flow restriction.71,72 A healthy autonomic nervous system of a bruxing child can fix the airway before it can be detected within the framework of a normal PSG.
Without esophageal pressure monitoring to demonstrate the increase respiratory effort, bruxism activity may not be recognized as being associated with a respiratory event.71,72 This RERA-related phenomenon was verified in a study of 50 pediatric subjects with an inclusion criteria of sleep-related tooth wear.73 No significant statistical association was found between AHI and the severity of bruxism. However, when respiratory effort–related arousals were added to the AHI, a statistically significant association was found. The bruxing events acted to protect the airway rather than to resolve an obstruction. Bruxism should rise with UARS, habitual snoring, and occasional snoring, because negative pressure and respiratory effort drives the action. The researchers concluded that pediatric sleep-related tooth wear could be used as a marker for SDB. Currently, no other healthcare provider is more equipped to evaluate and monitor pediatric nocturnal tooth wear than the dental practitioner (Figure 21 and 22).
Conclusion
The impact of SDB on the growing, snoring child can be serious, and sleep prosthodontics plays a unique role in screening and treating these patients. Dentists should screen children based on history and pediatric sleep questionnaire, as well as physical, intraoral, airway, and radiographic examination. It is important to note that dentists should not consider treatment in these children without a medical evaluation and possibly a sleep study. Interdisciplinary treatment options must be reviewed within the context of the particular sleep breathing disorder, age of the patient, and level of cooperation of the child and the parents. Given its focus on MAAs, sleep dentistry is limited in treating the snoring child. Sleep prosthodontics screens possible OSA/UARS in children and acts as a conduit of care, placing the patient in the proper medical, orthodontic, orthognathic, nutritionist/dietician, and/or oral mycology care for the best possible results. Dentistry has a bigger role to play in SDB, and sleep prosthodontics encapsulates that role.
References
1. Gill AI, Schaughency E, Galland BC. Prevalence and factors associated with snoring in 3-year olds: early links with behavioral adjustment. Sleep Med. 2012;13(9):1191-1197.
2. Kurnatowski P, Putyński L, Lapienis M, Kowalska B. Neurocognitive abilities in children with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2006;70(3):419-424.
3. Cho JG, Witting PK, Verma M, et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34(6):751-757.
4. Bao G, Guilleminault C. Upper airway resistance syndrome—one decade later. Curr Opin Pulm Med. 2004;10(60):461-467.
5. Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163(1):19-25.
6. Redline S. Morbidity, mortality, and public health burden of sleep apnea. In: McNicholas WT, Phillipson EA, eds. Breathing Disorders in Sleep. London: WB Saunders; 2002:222-235.
7. Chen W, Kushida CA. Nasal obstruction in sleep-disordered breathing. Otolaryngol Clin North Am. 2003;36(3):437-460.
8. Gold AR, Dipalo F, Gold MS, Broderick J. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27(3):459-466.
9. Woodson BT. Expiratory pharyngeal airway obstruction during sleep: a multiple element model. Laryngoscope. 2003;113(9):1450-1459.
10. Walter LM, Foster AM, Patterson RR, et al. Cardiovascular variability during periodic leg movements in sleep in children. Sleep. 2009;32(8):1093-1099.
11. Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862-865.
12. Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;19(3):CD001106.
13. Somiah M, Taxin Z, Keating J, et al. Sleep quality, short-term and long-term CPAP adherence. J Clin Sleep Med. 2012;8(5):489-500.
14. Tokunaga T, Ninomiya T, Kato Y, et al. Long-term compliance with nasal continuous positive airway pressure therapy for sleep apnea syndrome in an otorhinolaryngological office [published online ahead of print April 9 2013]. Eur Arch Otorhinolaryngol. http://link.springer.com/article/10.1007/s00405-013-2483-3#page-1. Accessed May 23, 2013.
15. Rouse JS. The bruxism triad: sleep bruxism, sleep disturbance, and sleep-related GERD. Inside Dentistry. 2010;6(5):33-44.
16. Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242-252.
17. Chervin RD, Clarke DF, Huffman JL, et al. School performance, race, and other correlates of sleep-disordered breathing in children. Sleep Med. 2003;4(1):21-27.
18. Guilleminault C, Khramtsov A. Upper airway resistance syndrome in children: a clinical review. Semin Pediatr Neurol. 2001;8(4):207-215.
19. Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34(7):875-884.
20. Piteo AM, Lushington K, Roberts RM, et al. Prevalence of snoring and associated factors in infancy. Sleep Med. 2011;12(8):787-792.
21. Archbold KH, Pituch KJ, Panahi P, Chervin RD. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140(1):97-102.
22. Ivanenko A, Crabtree VM, Obrien LM, Gozal D. Sleep complaints and psychiatric symptoms in children evaluated at a pediatric mental health clinic. J Clin Sleep Med. 2006;2(1):42-48.
23. Li AM, Au CT, Ng SK, et al. Natural history and predictors for progression of mild childhood obstructive sleep apnoea. Thorax. 2010;65(1):27-31.
24. O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13(2):165-172.
25. Barnes ME, Huss EA, Garrod KN, et al. Impairments in attention in occasionally snoring children: an event-related potential study. Dev Neuropsychol. 2009;34(5):629-649.
26. Blunden S, Lushington K, Lorenzen B, et al. Neuropsychological and psychosocial function in children with a history of snoring or behavioral sleep problems. J Pediatr. 2005;146(6):780-786.
27. O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44-49.
28. Urschitz MS, Eitner S, Guenther A, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114(4):1041-1048.
29. Lopes MC, Guilleminault C. Chronic snoring and sleep in children: a demonstration of sleep disruption. Pediatrics. 2006;118(3);e741-e746.
30. Barnes ME, Huss EA, Garrod KN, et al. Impairments in attention in occasionally snoring children: an event-related potential study. Dev Neuropsychol. 2009;34(5):629-649.
31. Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129(4):e857-e865.
32. Gozal D, Pope DW Jr. Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107(6):1394-1399.
33. Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol. 2011;46(4):313-323.
34. Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51(5):416-433.
35. Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol. 2011;178(3):465-474.
36. Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front Neurol. 2012;3:7.
37. Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126(5):e1161-e1167.
38. Spicuzza L, Leonardi S, La Rosa M. Pediatric sleep apnea: early onset of the ‘syndrome’? Sleep Med Rev. 2009;13(2):111-122.
39. Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254-259.
40. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(5):576-584.
41. Guilleminault C, Khrammtsov A. Upper airway resistance syndrome in children: a clinical review. Semin Pediatr Neurol. 2001;8(4):207-215.
42. Enlow DH, Hans MG. Basic growth concepts. In: Enlow DH, Hans MG, eds. Essentials of Facial Growth. Philadelphia, PA: WB Saunders Company; 1996:18-38.
43. Huang YS, Guilleminault C. Pediatric obstructive sleep apnea and the critical role of oral-facial growth: evidences. Front Neurol. 2012;3:1-7.
44. Kim JH, Guilleminault C. The nasomaxillary complex, the mandible, and sleep-disordered breathing. Sleep Breath. 2011;15(2):185-193.
45. Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122(3):840-851.
46. Ruoff CM, Guilleminault C. Orthodontics and sleep-disordered breathing. Sleep Breath. 2012;16(2):271-273.
47. Juliano ML, Machado MA, de Carvalho LB, et al. Polysomnographic findings are associated with cephalometric measurements in mouth-breathing children. J Clin Sleep Med. 2009;5(6):554-561.
48. Harvold EP, Tomer BS, Vargervik K, Chierici G. Primate experiments on oral respiration. Am J Orthod. 1981;79(4):359-372.
49. Solow B, Skov S, Ovesen J, et al. Airway dimensions and head posture in obstructive sleep apnoea. Europ J Orthod. 1996;18(6):571-579.
50. Güray E, Karaman AI. Effects of adenoidectomy on dentofacial structures: a 6-year longitudinal study. World J Orthod. 2002;3(1):73-81.
51. Guilleminault C, Stoohs R, Kim YD, et al. Upper airway sleep-disordered breathing in women. Ann Intern Med. 1995;122(7):493-501.
52. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(5):576-584.
53. Zettergren-Wijk L, Forsberg CM, Linder-Aronson S. Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnoea—a 5-year follow-up study . Eur J Orthod. 2006;28(4):319-326.
54. Guilleminault C, Monteyrol PJ, Huynh NT, et al. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath. 2011;15(2):173-177.
55. Oliveira De Felippe NL, Da Silveira AC, Grace Viana G, et al. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: short- and long-term effects. Am J Orthod Dentofacial Orthop. 2008;134(3):370-382.
56. Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 2004;27(4):761-766.
57. Lavigne GJ, Manzini C, Kato T. Sleep Bruxism. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. Philidelphia, PA: Saunders; 2005:946-959.
58. Carlsson GE, Egermark I, Magnusson T. Predictors of bruxism, other oral parafunctions, and tooth wear over a 20-year follow-up period. J Orofac Pain. 2003;17(1):50-57.
59. van Selms MK, Lobbezoo F, Wicks DJ, et al. Craniomandibular pain, oral parafunctions, and psychological stress in a longitudinal case study. J Oral Rehabil. 2004;31(8):738-745.
60. Pierce CJ, Chrisman K, Bennett ME, Close JM. Stress, anticipatory stress, and psychologic measures related to sleep bruxism. J Orofac Pain. 1995;9(1):51-56.
61. Seraidarian P, Seraidarian PI, das Neves Cavalcanti B, et al. Urinary levels of catecholamines among individuals with and without sleep bruxism. Sleep Breath. 2009;13(1):85-88.
62. Zhang J, Ma RC, Kong AP, et al. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34(2):225-233.
63. Gastaldo E, Quatrale R, Graziani A, et al. The excitability of the trigeminal motor system in sleep bruxism: a transcranial magnetic stimulation and brainstem reflex study. J Orofac Pain. 2006;20(2):145-155.
64. Lavigne GJ, Khoury S, Abe S, et al. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35(7):476-494.
65. Khoury S, Rouleau GA, Rompré PH, et al. A significant increase in breathing amplitude precedes sleep bruxism. Chest. 2008;134(2):332-337.
66. Miyawaki S, Lavigne GJ, Pierre M, et al. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep. 2003;26(4):461-465.
67. Kato T, Montplaisir JY, Guitard F, et al. Evidence that experimentally induced sleep bruxism is a consequence of transient arousal. J Dent Res. 2003;82(4):284-288.
68. Krieger J. Breathing in normal subjects. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, PA: WB Saunders Company; 2000:229-241.
69. Carra MC, Rompré PH, Kato T, et al. Sleep bruxism and sleep arousal: an experimental challenge to assess the role of cyclic alternating pattern. J Oral Rehabil. 2011;38(9):635-642.
70. Kato T, Thie NM, Huynh N, et al. Topical review: sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;17(3):191-213.
71. Simmons JH, Prehn R. Airway protection: the missing link between nocturnal bruxism and obstructive sleep apnea. Paper presented at: 23rd Annual Meeting of the Associated Professional Sleep Societies; June 6-11, 2009; Seattle, WA. Abstract A218.
72. Simmons JH, Prehn R. Nocturnal bruxism as a protective mechanism against obstructive breathing during sleep. Paper presented at: 22rd Annual Meeting of the Associated Professional Sleep Societies; June 7-12, 2008; Baltimore, MA. Abstract A199.
73. Singh N, Chandwani B, Finkelmann M, et al. Sleep bruxism-related tooth wear as a clinical marker for pediatric sleep-disordered breathing. Paper presented at: 21st Annual American Academy of Dental Sleep Medicine Meeting; June 8, 2012; Boston, MA. Poster #015.
About the Author
Jeffrey S. Rouse, DDS
Private Practice Prosthodontist
San Antonio, Texas