You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The loss of the dentoalveolar complex secondary to disease, trauma, or congenital deformity can lead to significant functional, esthetic, emotional, and psychological sequelae for many people. The ability to predictably regenerate these lost tissues has been a challenge for all dental clinicians.
Regenerative treatment approaches in medicine and dentistry have historically included the use of three-dimensional biomaterial matrices to support the regeneration of tissues lost to disease, trauma, or congenital deformity. Autogenous bone grafts used in oral, periodontal, and craniofacial bone-grafting procedures are still considered the gold standard because they provide all of the elements necessary for bone repair and remodeling: undifferentiated and osteocompetent cells, growth-stimulating molecules, and an osteoconductive matrix. There is, however, potential variability associated with the quality of this autogenous bone depending on the relative health status of the patient. Inevitably, patients with the greatest need for bone regeneration often present with problems such as diabetes, osteoporosis/osteopenia, and/or a smoking history that could lead to lesser autogenous bone quality. There are disadvantages of autogenous bone harvesting from even healthy patients that include limited volume, increased donor site morbidity, increased postoperative pain, and longer procedure time. In some instances the disadvantages may well outweigh the advantages of this procedure.
As a result of the limitations of autogenous bone grafts, a variety of substitutes have been used as matrices in oral surgery and periodontal procedures. Some of these include allografts, xenografts, and synthetic materials that function primarily in a passive mode by guiding or conducting cell migration through the matrix, resulting in eventual repair of the defect. These matrices can be used alone or in combination in procedures such as grafting defects associated with extractions, periodontal disease, periapical infection, implant placement/repair, or inadequate alveolar bone width and/or height. In addition, these materials may be used alone or in combination with autogenous bone, or in combination with various mesh (resorbable and nonresorbable), barrier membranes (GBR), or other passive materials designed to act as a physical barrier for cells involved in the regenerative process. These non-autogenous grafting options provide a means to maintain space as well as a framework for tissue deposition in a passive mode. However, results can be variable depending on the host health response as well as the inherent physical and chemical properties of the material themselves.
Tissue-Engineering Perspective
The problem of obtaining predictable outcomes when using passive therapeutic approaches such as guided bone regeneration and osteoconductive matrices has led to the development of treatment modalities specifically designed to stimulate the cells responsible for bone regeneration. This tissue-engineering approach to bone and periodontal regeneration combines three key elements to augment regeneration: osteocompetent cells, signaling molecules, and conductive scaffolds (Figure 1).1 A number of naturally occurring bioactive proteins (signaling molecules) are known to be present in bone, platelets, and a number of other cells and tissues. Two types of molecules have received the greatest attention and include growth factors that are primarily mitogenic (cell-proliferative) and chemotactic (cell-recruiting) agents and morphogens that act by causing the differentiation of stem cells into bone forming cells—a process also known as osteoinduction. The purpose of this article is to cover general tissue-engineering strategies, principles, and clinical applications of recombinant proteins to implant dentistry.
This exciting discipline uses the knowledge base of cell biology, molecular biology, and biochemistry for the growth and development of new tissues. It also draws on advances in materials sciences and engineering to help design functional tissues. In addition, this field of research incorporates the therapeutic principles of medical and dental clinicians (and surgeons) in order to apply the scientific element to practical application.2 Tissue engineering builds on the interface between materials, science, and biocompatibility, and integrates cells, natural or synthetic scaffolds, and specific signals to create new tissues.3
Tissue-Engineering Strategies
A number of strategies have evolved to engineer new tissues, and most combine a material with either bioactive molecules that induce tissue formation or cells grown in the laboratory. Typically the bioactive molecules are growth-factor proteins that are involved in natural tissue formation and remodeling. This approach is predicated on the local delivery of an appropriate growth factor at a correct dose for a defined period of time that, in turn, will allow the recruitment, proliferation, and differentiation of a patient’s cells from adjacent tissues. These cells are then able to play a key role in tissue repair and/or regeneration at the specific site.
A second tissue-engineering strategy involves direct transplantation of cells grown in the laboratory. These cells are usually derived from a small tissue biopsy specimen and expanded in the laboratory to allow new tissue to be engineered that will be formed in part from the transplanted cells. Specific materials are used to deliver the molecules or cells to the appropriate anatomic site and provide mechanical support to the forming tissue by acting as a scaffold to guide new tissue formation.4 These biomaterials are approved for medical use by the US Food and Drug Administration. The most widely used synthetic materials are polymers of lactide and glycolide.5 Both of these polymers have a long history of human use, are biocompatible, and possess physical properties such as degradation rates and mechanical strengths that are easily manipulated. Type 1 collagen, a natural polymer, is commonly used because of its biocompatibility and ability to be remodeled by cells.
Three general approaches are commonly used to engineer tissues: conductive, inductive, and cell transplantation. Conductive approaches involve biomaterials in a passive mode to facilitate the growth or regenerative capacity of existing tissue. The most widespread use of this involves the concept of osseointegration of a dental implant. This initial application by Brånemark et al is relatively simple in that the implants used do not include living cells or diffusible biological signals.6,7 Today, the use of implants in dentistry is widespread and considered a standard treatment option in replacing missing teeth in both partially and completely edentulous patients. Still another straightforward example of using biomaterials in a passive mode to facilitate the growth or regenerative capacity of existing tissue is the use of barrier membranes in guided tissue regeneration. Nyman et al were the first to successfully use osteoconductive barriers to provide a means for selective wound healing by supporting a means for selective wound healing by supporting the ingrowth of the periodontal cells, while excluding gingival epithelium and connective tissue cells from reconstruction sites.8 This guided tissue-regeneration approach is most often used to regenerate the periodontal supporting structures and uses a barrier membrane to create a protected compartment for wound healing.9
A second tissue-engineering approach involves tissue induction that activates cells near the targeted tissue with specific biologic signals. The origins of this method stem from the discovery of bone morphogenetic proteins (BMPs). Urist first showed that new bone could be formed at non-mineralizing, or ectopic, sites after implantation of powdered demineralized bone.10 The BMPs, contained within the demineralized powdered bone, were found to be the proteins responsible for inducing bone formation. These same proteins have become readily available in recombinant forms and are produced on a large scale by biotechnology companies. Another method of using diffusible growth factors involves placing specific extracellular matrix molecules on a scaffold support at a tissue site. These molecules are able to direct the function of cells already present at that site, and promote the formation of a specific tissue type. An example of this is the use of an enamel protein preparation of pig origin to promote new bone formation in periodontal defects.11
The third tissue-engineering approach is one that addresses a primary limitation of the inductive approach, namely, that the inductive factors for a particular tissue may not be known. Here, cell transplantation can be very appealing. This approach involves direct transplantation of cells grown in the laboratory.12 One of the most successful applications of cell transplantation involves the development of a tissue-engineered skin equivalent.13 Skin tissue is widely needed to treat burn victims and patients with diabetic ulcers. These needs led to early research on the engineering of skin tissues, and resulted in the first FDA-approved tissue-engineered products for clinical use.14,15
Tissue-Engineering Principles and Osseointegration
As previously mentioned, there are three key elements that must be present and work synergistically to promote tissue regeneration: osteocompetent cells, signaling molecules, and conductive scaffolds (Figure 1).1 The cells include pluripotential stem cells, as well as cells that are only partially differentiated along their lineage. The signals are growth factors such as platelet-derived growth factor AA, AB, and BB (PDGF-AA; PDGF-AB; PDGF-BB), several forms of transforming growth factor-β (TGF- β) including a number of BMPs, vascular endothelial growth factor, epidermal growth factor, and others.16 These growth factors act on the external cell membrane receptors of the target cell to stimulate the expression of a normal gene.17 The matrix acts as a scaffold on which tissue is grown and allows cells to migrate upon itself. Many clinicians use a variety of materials for hard-tissue regeneration, including allogeneic bone and xenogeneic bone, which are osteoconductive and act as a type of matrix. The process of osseointegration represents a good example of tissue engineering. A carefully prepared drill site is created before dental implant placement. The osteotomy exposes bone marrow that contains endosteal osteoblasts, stem cells, and blood that contains platelets. Once the implant is mechanically fixated, a blood clot forms that permeates within the interface between the implant surface and the bone. Next, platelet degranulation occurs, which results in the release of PDGF-AA, PDGF-AB, PDGF-BB, TGF-β1, TGF- β2, vascular endothelial growth factor, epidermal growth factor, and vitronectin, a key cell adhesion molecule. The clotted blood plasma also deposits fibrin and fibronectin onto the implant surface and forms a connecting bridge between the bony wall and the implant surface.19
Next, the released growth factors act on the exposed marrow cells, stem cells, endothelial cells, and endosteal osteoblasts to create an angiogenic and mitogenic effect in addition to cellular migrations and differentiation. The endosteal osteoblasts then begin to lay down osteoid on the cell adhesion molecules fibrin, fibronectin, and vitronectin, and proceed to migrate toward the implant surface by displacing daughter cells onto their own surface as they undergo mitosis. These daughter cells (osteoblasts) then continue to lay down bone onto the cell adhesion molecules as they become differentiated.19 These cells continue to follow the cell adhesion molecules, which become attached to the implant surface. Ultimately, they become fully differentiated and begin to secrete sialoprotein and osteopontin onto the cell adhesion molecule strands that are deposited onto the implant surface. Osseointegration of the implant ultimately occurs when the osteoblasts insert collagen into the cementing substance and forms bone attached to it.18,19
Recombinant Proteins
Advances in recombinant technology have allowed growth factors that can be synthesized, concentrated, purified, and packaged under tightly controlled and regulated conditions. Combination products also have been created that combine tissue-specific matrices with highly concentrated bioactive proteins that actively recruit regenerative competent cells to the treatment site and increase their cell numbers. This ability to combine highly purified concentrated signaling proteins with respective conductive matrices represents one of the most significant advances in tissue engineering.1 Two recombinant growth factors that have become widely commercialized for use in tissue regeneration in dentistry include rhPDGF-BB (with β tricalcium phosphates) and rhBMP-2 (with type 1 collagen sponge).
Recombinant human PDGF-BB (in gel formulation) became the first recombinant therapeutic protein approved by the FDA in 1997. In 2005 a combined product, rhPDGF-BB and β TCP was FDA-approved for bone and periodontal regeneration and treatment of gingival recession. This concentrated growth factor was found to have a concentration one thousand times that found through platelet-concentration techniques.20-22 Human histologic studies evaluating rhPDGF-BB in combination with bone allografts for the treatment of severe periodontal intrabony and Class II furcation defects provided the highest level of proof for true periodontal regeneration.1,23,24 A large-scale, randomized, controlled, blinded clinical trial using rhPDGF-BB to treat periodontal defects demonstrated this protein to be a potent stimulator of alveolar bone.25 Still another example of the advantage of bioactive recombinant therapeutics over passive wound healing techniques such as guided bone regeneration is a proof of principle study that evaluated vertical ridge augmentation in a standardized model using rhPDGF-BB in combination with block form anorganic bovine bone. The results of this study demonstrated the potential to regenerate significant amounts of new bone around dental implants placed in severely atrophic mandibular ridges.26
In 1965, Urist made the key discovery that demineralized, lyophilized extracts of rabbit bone induce new bone in intramuscular sites via a process he later described as autoinduction.10 Subsequently, BMPs were isolated from adult bone matrix in mammals.27 The human genome encodes 20 BMPs.27 The BMPs are dimeric molecules and are dependent on the single intermolecular disulfide bone for biologic activity. A single recombinant BMP can have pleiotropic effects on different steps in bone morphogenesis depending on its concentration: femtomolar concentrations promote chemotaxis, higher concentrations promote mitogenesis and differentitation.28 The BMPs play key roles in the development of the brain, eyes, heart, kidneys, skin, bones, and teeth. Their actions also include the formation of skeletal tissue during embryogenesis, growth and remodeling, and the induction and creation of new bone. In the postnatal skeleton, BMPs are intimately associated with the collagenous extracellular matrix and are localized in periosteal cells and in mesenchymal cells of marrow stroma during fracture repair.28 In addition, rhBMP-2 can induce bone de novo, ie, without the need for autogenous, allogeneic, xenogeneic, or bone-substitute products.
The combination of rhBMP-2 on an absorbable collagen sponge (ACS) carrier has been shown to induce bone formation in a number of preclinical and clinical studies. In 2002, rhBMP-2/ACS at a 1.5-mg/cc concentration (INFUSE® Bone Graft, Medtronic Spinal and Biologics, www.medtronic.com) was FDA-approved as an autograft replacement for certain spinal fusion procedures.19 In 2004, it was approved for open tibial fractures with an intermedullary nail fixation.29 It was also approved in 2007 as an alternative to autogenous bone grafts for maxillary sinus augmentation30 and for localized alveolar ridge augmentation for defects associated with extraction sockets.31
Case 1
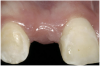
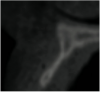
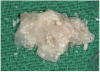
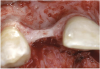
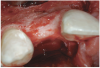
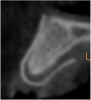
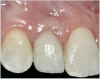
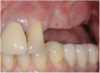
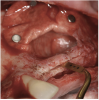
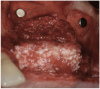
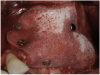
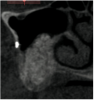
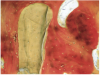
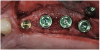
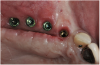
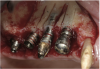
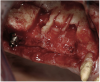
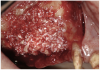
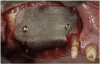
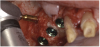
A 16-year-old female patient with a congenitally missing maxillary right lateral incisor was referred for an implant-supported crown. Clinical and CBCT evaluation revealed both a facial and palatal bony width deficiency (Figure 2 and Figure 3). The treatment plan included the use of titanium mesh and rhBMP-2/ACS (INFUSE® Bone Graft) along with mineralized allograft (MinerOss®, BioHorizons, www.BioHorizons.com) for bony augmentation (Figure 4 and Figure 5). After 7 months, the graft was found to be well incorporated and allowed for placement of a 3.8-mm x 10.5-mm diameter root form implant that was restored 3 months later (Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10).
Case 2
A 62-year-old woman was referred for fixed tooth replacement of the missing maxillary left cuspid, bicuspids, and first molar (Figure 11). Clinical and CBCT evaluation revealed an almost fully pneumatized maxillary left sinus along with alveolar width deficiency. The treatment plan called for a four-unit, implant-supported splinted fixed bridge. A left sinus augmentation along with a ridge split and interpositional bone graft consisting of rhBMP-2/ACS (INFUSE Bone Graft) and mineralized allograft (MinerOss) covered by a dense porous polyethylene mesh (Medpor®, Stryker, www.stryker.com) were done simultaneously (Figure 12, Figure 13 and Figure 14). Six months later, a follow-up CBCT revealed a well-incorporated graft with adequate bone volume and density for implant placement (Figure 15 and Figure 16). Final bridge fabrication followed 3 months later (Figure 17 and Figure 18).
Case 3
A 72-year-old man was referred for removal of failing maxillary right posterior implants (Figure 19). The treatment plan included removal of three implants with simultaneous bone augmentation and replacement implants for implant-supported fixed bridgework. Implant removal and bone grafting with rhPDGF (Gem 21®, Osteohealth, www.osteohealth.com) and allograft (MinerOss) and xenograft (BioOss®, Geistlick, www.geistlickonline.com) were used along with titanium mesh for space maintenance (Figure 20, Figure 21 and Figure 22). Six months later, mesh removal revealed type I bone allowing for placement of three implants (Figure 23 and Figure 24).
Conclusion
Growth factors are generally accepted to be essential mediators of tissue repair via well established mechanisms of action that include stimulatory effects on angiogenesis and cell proliferation, ingrowth, differentiation, and matrix biosynthesis. Several studies that include rhPDGF and rhBMP-2 have been done that demonstrate the safety and efficacy of these recombinant proteins with regard to their ability to regenerate bone for dental implant reconstruction.
In a large randomized controlled trial, Nevins et al demonstrated the safety and effectiveness of using rhPDGF-BB mixed with a synthetic beta-tricalcium phosphate (β-TCP) matrix for the treatment of advanced periodontal osseous defects at 6 months of healing. The effectiveness measurements of this 180-subject study included clinical attachment level and gingival recession measured clinically, and linear bone growth and percent bone fill as assessed radiographically. The study demonstrated that the use of rhPDGF-BB simulated a significant increase in the rate of clinical attachment level gain, reduced gingival recession 3 months after surgery, and improved bone fill as compared to β-TCP substitute at 6 months.32
Choo et al33 examined the effect of a combination of rhPDGF-BB mixed with a synthetic β-TCP on bone healing around dental implants with critical size circumferential defects prepared in the ilium of six sheep. Three dental implants were placed in each defect and the implant bone gap was filled with blood clot only, β-TCP only, or rhPDGF-BB (0.3 mm/ml) and β-TCP. All defects were covered with a resorbable barrier membrane. The combination of PDGF-BB with β-TCP resulted in enhanced bone regeneration in all of the peri-implant bone defects.
Fiorellini et al documented the efficacy of rhBMP-2/ACS in fresh extraction sockets with missing buccal plates and showed superior bone regeneration in socket width and bone regeneration at the crest compared with the results in an unfilled extraction socket and a socket filled with the collagen sponge alone. These findings were consistent with proof of principal de novo bone formation. This de novo bone was superior at all levels of the socket resulting in a superior hard-tissue environment for implant placement. Implant survival at 2 years was 67% for the rhBMP2/ACS socket and 38% and 50% for the unfilled socket and collagen sponge sockets, respectively.31
In a multicenter, prospective, randomized clinical study, Triplett et al evaluated the safety and effectiveness of rhBMP-2/ACS compared with an autogenous bone graft for 2-stage maxillary sinus augmentation. The results showed significant new bone formation by 6 months in each group. The mean change in bone height in the rhBMP-2/ACS group was 7.83 mm +/- 3.52 mm versus 9.46 mm +/- 4.11 mm for the autogenous bone graft group. At 6 months post-prosthetic loading, the rhBMP-2/ACS subjects had significantly denser bone than those in the autogenous bone graft group. No differences were found in the histologic parameters evaluated between the two groups. The success rate for the rhBMP-2/ACS group was 79% (64 of 81 subjects), and 201 of 251 implants placed in the autogenous bone group and 199 of 241 implants placed in the rhBMP2-ACS group were integrated, retained, and functional at 6 months post-loading. Most importantly, the implants placed in both groups performed similarly after functional loading.30
New technology has traditionally had a major impact in dentistry, from the development of computer-based technologies to restorative materials. Tissue engineering, as an interdisciplinary field, will continue to bring the power of modern biological, chemical, and physical science to everyday clinical problems. Our understanding of the respective biologic and physical requirement to achieve predictable tissue-specific regeneration will continue to evolve as more properties of the bioactive proteins, conductive cell scaffolds, and the application of stem cells to effectively treat degenerative tissue (and organ) conditions are discovered. Further controlled clinical trials are necessary to establish the effectiveness of the two recombinant proteins that are currently being used (rhPDGF-BB and rhBMP-2/ACS) with regard to their degree of influence on treatment success with dental implant reconstruction.
References
1. Lynch S. Introduction. In Lynch S, et al. Tissue Engineering Applications in Oral and Maxillofacial Surgery and Periodontics, 2nd ed. Hanover Park, IL: Quintessence Publishing Co, Inc.; 2008.
2. Lanza RP, Langer R, Chick WL, eds. Principles of Tissue Engineering. San Diego, CA: Academic Press, Inc; 2000:3-5.
3. Mooney DJ, Rowley JA. Tissue engineering: integrating cells and materials to create functional tissue replacements. In: Park K, ed. Controlled Drug Delivery. Washington, DC: ACS Books; 1997:333-346.
4. Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16(5):224-230.
5. Kim BS, Nikolovski J, Bonadio J, et al. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Exp Cell Res. 1999;251(2):318-328.
6. Brånemark P, Breinen U, Adell R, et al. Intraosseous anchorage of dental prostheses: experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81-91.
7.Albrektsson T, Hanson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985;6(2):97-101.
8. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290-296.
9. Caudill RF. Guided bone regeneration and implants: history and case reports. In: Block MS, Kent JM, Guerra LR, eds. Implants in Dentistry. Philadelphia, PA: Saunders; 1997:183-91.
10. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
11. Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (Emdogain) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24(9 Pt 2):705-714.
12. Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10(2):165-181.
13. Morrison G. Advances in the skin trade. Mechanical Eng. 1999;121:40-43.
14. Parenteau N. The organogenesis story. Sci Am. 1999;280(4):83-84.
15. Naughton G. The advanced tissue sciences story. Sci Am. 1999;280(4):84-85.
16. Marx RE, Garg AK. The biology of platelets and the mechanism of platelet-rich plasma. In: Dental Craniofacial Applications of Platelet-Rich Plasma. Chicago, IL: Quintessence; 2005:3-30.
17. Kratchmarova I, Blagoev B, Haack-Sorensen M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472-1477.
18. Davies JE, Lowenberg B, Shiga A. The bone-titanium interface in vitro. J Biomed Mater Res. 1990;24(10):1289-1306.
19. Marx R. Application of tissue engineering principles to clinical practice. In Lynch S, et al. Tissue Engineering Applications in Oral and Maxillofacial Surgery and Periodontics, 2nd ed. Hanover Park, IL: Quintessence Publishing Co, Inc.; 2008:47-63.
20. Bowen-Pope DF, Malpass TW, Foster DM, Ross R. Platelet-derived growth factor in vivo: Levels, activity, and rate of clearance. Blood. 1984;64(2):458-469.
21. Huang JS, Huang SS, Deuel TF. Human platelet-derived growth factor: Radioimmunoassay and discovery of a specific plasma-binding protein. J Cell Biol. 1983;97(2):383-388.
22.Singh JP, Chaikin MA, Stiles CD. Phylogenetic analysis of platelet-derived growth factor by radio-receptor assay. J Cell Biol. 1982;95(2 Pt 1):667-671.
23. Nevins M, Camelo M, Nevins ML, et al. Periodontal regeneration in humans using recombinant human platelet-derived growth factor BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74(9):1282-1292.
24. Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human class II furcations using purified recombinant human platelet-derived growth factors-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213-225.
25. Nevins M. Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205-2215.
26. Simion M, Rocchietta I, Kim D, et al. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: A histologic study in a dog model. Int J Periodontics Restorative Dent. 2006:26(5):415-423.
27. Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247-252.
28. Bostrom MP, Lane JM, Berberian WS, et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13(3):357-367.
29. Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic Protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A(12):2123-2134.
30. Triplett GP, Nevins M, Marx RE, et al. Pivotal randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67(9):1947-1960.
31. Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76(4):605-613.
32. Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205-2215.
33. Choo T, Marino V, Mark Bartold P. Effect of PDGF-BB and beta-tricalcium phosphate (β-TCP) on bone formation around dental implants: a pilot study in sheep. Clin Oral Implants Res. 2011 Nov 22. doi: 10.1111/j.1600-0501.2011.02345.x. [Epub ahead of print.]
For additional content on Bone Grafting and Implant Placement, visit: dentalaegis.com/go/id102
About the Author
Michael A. Pikos, DDS
Founder and CEO
Pikos Implant Institute
Palm Harbor, Florida
Founder
Coastal Jaw Surgery
Palm Harbor, Florida