You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Many practitioners subscribe to myths about pain related to local anesthetic injection and the potential of using topical anesthesia as a mitigating factor. Examination of evidence shows both the fallacy and accuracy of aspects of these myths. Increased knowledge concerning anesthesia can make dental techniques more effective and less painful, which can help in practice building as patients will come to see the informed clinician as someone who provides virtually painless dentistry.
Myth #1: "Use of topical anesthetic helps decrease injection pain."
Common topical anesthetics include benzocaine, tetracaine, lidocaine, prilocaine, and a eutectic mixture of local anesthetics lidocaine and prilocaine (EMLA). Some are delivered through a blunt-end needle or via a topical application of gel, while others are delivered via spray.
Data is contradictory as to their efficacy in reducing injection pain. Some studies indicate that a topical can reduce needle insertion pain, but others do not demonstrate any difference between a topical and a placebo. A predominance of the literature says a topical anesthetic can help."1-4
Some studies indicated that application time is important; generally the time recommended is 1 to 2 minutes, but may be up to 10. High concentration topicals may have better efficacy, but also offer potentially higher absorption into systemic circulation, so those should be limited to 2 minutes or less.
Effectiveness may be different based on the region of the oral cavity anesthetized; more effective in the anterior maxilla, including palatal, but less effective in the posterior maxilla or inferior alveolar (IA) area.
Some studies show improvement when switching from benzocaine to EMLA; others do not. Most literature does agree that topical anesthetics only can provide relief of pain from needle puncture, and do not affect pain from local anesthetic deposition.
In a 2019 study by Rehman and Qazi featuring 100 subjects (49 male and 52 female), using 20% benzocaine, application time 1 minute, 0.6 mL of local anesthetic injected, pain was evaluated on a Visual Analog Scale of zero (no pain) to 10 (worst pain).5Participants rated pain twice: during insertion, and again during disposition. Results showed that although 20% benzocaine significantly reduced pain on needle penetration during buccal infiltration in maxillary anterior teeth, the difference compared to when no topical was used was small and the clinical significance not clear. Topical anesthetic did not affect pain of local anesthetic disposition.
The authors broke reactions down by gender; fear of dental settings; phobia of needles; and smoking habits. They found a small but significant pain reduction using benzocaine vs. no benzocaine; a significant difference in males vs females, with women experiencing more pain; more pain among those fearing the dental setting and those with phobia of needles vs without; and more pain among non-smokers (Figure 1).
This information can help make the patient experience better; rather than indiscriminately using topical for every injection, instead the practitioner may want to prioritize the use of topical anesthesia on women, the fearful or phobic, and non-smokers.
The Food and Drug Administration (FDA) issued a warning in May 2018 noting that exposing children age 2 or younger to benzocaine could pose a serious risk of methemoglobinemia.6 This is reinforced by the Pennsylvania Patient Safety Authority, which earlier had issued an advisory noting the "condition can be life-threatening, potentially causing cyanosis, confusion, hemodynamic instability, and coma if not recognized and treated properly."7 The topical anesthetic agent, particularly benzocaine and prilocaine, creates a change in hemoglobin that prevents the carrying of oxygen. Use of topicals, therefore, is strongly cautioned in young children.
For high-concentration topicals, the practitioner must keep application time in mind because the more the topical is in contact with tissue, the greater the potential for higher systemic absorption.
So while the conclusion concerning topicals decreasing injection pain is unclear, practitioners can achieve best results by taking into account patient experience, expectation, and age, and using topicals for the correct application time.
Myth #2: "Local anesthetic without epinephrine stings less"
Some practitioners believe that by using a plain local anesthetic solution of 3% mepivacaine and 4% prilocaine with no epinephrine ("epi"), the patient will not feel a pinch, sting, or burn at injection because the pH will be higher-closer to a normal body pH of approximately 7.37-as solutions containing a vasoconstrictor such as epi have a lower pH than plain solutions.
A study performed by this author and a colleague, in which we examined all available solutions (as opposed to brands) on the US market, discovered that the average pH of solutions containing epi is 3.82, and the average pH of plain solutions is 6.34, reinforcing the idea that there is a difference in the two types of solutions, and that the difference in injection pain could be the result of solution pH.8
Another study by Kramp et al evaluated three local anesthetic solutions: 2% lidocaine with 1:100,000 epinephrine, pH 4.12; 2% mepivacaine with 1:20,000 levonordefrin, pH 3.05; and 4% prilocaine, pH 6.28, on 100 patients and 100 injections with a 30-gauge needle, with slow injection speed and no topical. In general, it can probably be said that slow injection technique (one cartridge delivered over 60 seconds) results in better local anesthetic success and less discomfort compared to fast injection technique (one cartridge delivered over approximately 15 seconds).9
The results show that in three out of four injection types, prilocaine, the solution with the highest pH, was least painful.10
In a study by Wahl et al, four solutions-4% prilocaine, 3% mepivacaine, 4% articaine with 1:100,000 epinephrine, and 2% lidocaine with 1:100,000 epinephrine-were compared by two dentists on 1,391 patients using a 25-gauge needle and a topical anesthetic. Based solely on the higher pH of prilocaine and mepivacaine the expectation is that these solutions should be equivalent with respect to injection pain, and less painful then the solutions containing epinephrine. Yet, prilocaine again was associated with the least amount of injection pain, and mepivacaine was associated with the worst pain, leading one to believe that the cause of prilocaine causing the least amount of injection pain is the result of more than just pH.11
Another study by Wahl et al compared 4% prilocaine plain (pH 6-7) to 0.5% bupivacaine with epinephrine (pH 3.3-3.5), with 681 injections provided by two dentists using a 25-gauge needle and topical anesthetic. Prilocaine again was less painful.12
The verdict, then, is confirmation that based on the type of solution used by the practitioner, he or she can reduce injection pain by first providing a local anesthetic injection using prilocaine, and then administering other solutions if additional anesthetic time is required. It is still unclear why prilocaine, even compared to other local anesthetic solutions without epinephrine, produces the least injection pain. Another interesting fact about prilocaine is that it has a favorable safety profile when administered to pregnant patients.13
Myth #3: "Warming the local anesthesia cartridge before injection increases patient comfort."
In 1920, Dr. Harvey Cook, after treating patients during World War I, designed a glass vial in the shape of a rifle cartridge, employing a pencil eraser as a stopper, and used a brass syringe and double-ended needle to prepare procaine injections. In 1925, he patented this as the Carpule System. The terms "cartridge" and "carpule" are considered interchangeable today, but technically cartridge is the correct term.
Some researchers recommend warming, while others believe it has no benefits, and it is still unclear exactly why warming may work. Some suggested reasons involve changes in nociception, based on the belief that cold is more painful than warm; lowering of the pKa to create a more base form of the anesthetic; or increased solubility, expanding the anesthetic's ability to pass across the lipid membrane.
Practitioners often use low-tech methods for warming up a dental local anesthetic cartridge before injection-holding it in the hand to employ body heat or placing in in a cup of warm water. Another possibility are cartridge warming devices to achieve a recommended temperature for the warmed cartridge contents of 37 to 43°C (Figure 2).
This author tested the Premier Dental warming device by placing a meat thermometer within, close to the 3-watt light bulb where cartridges are held, and the temperature was 40.5°C, showing that the device will warm the cartridge to the appropriate temperature.
Some data indicate that the clinical onset and duration may be affected by the temperature. A study by Dabarakis et al of plain 3% mepivacaine showed a difference in duration: cooled anesthetic actually provided a statistically significant longer duration of pulpal anesthesia than anesthetic at room temperature.14
A study by Hogan et al showed that warming local anesthetics leads to less pain during injection, and therefore was indicated before administration. The meta-analysis also found a reduction in pain from warmed anesthetics whether they were injected subcutaneously or intradermally. No significant benefit was observed for intraoral injection.15 However, that conclusion may be incomplete, as much of the study was conducted outside the mouth, with only one portion included that observed the intraoral injection.
One study on skin by Lundbom et al had 36 healthy volunteers receive three injections of 4.5 mL 1% lidocaine subcutaneously into the abdomen, one refrigerated at 8°C, one at room temperature at 21°C, and one warmed to 37°C.16Patients rated their pain; warmed/heated local anesthetic was the least painful; cooled was intermediate; and the most painful was room temperature-the temperature at which most practitioners provide local anesthetics. The study showed that warming, and even cooling, are better than room temperature.
In a split-mouth randomized clinical trial led by Aravena et al in which each of 72 patients received either a warmed or room-temperature injection of 0.9 mL of 2% lidocaine with 1:100,000 epi in the buccal vestibule in the maxillary lateral incisal area, results showed that warmed local anesthesia resulted in significantly reduced pain compared to room temperature injections.17
So while the verdict technically is unclear concerning warming cartridges because the exact mechanism is unknown, there is no downside to warming local anesthetics, and doing so tends to make anesthetic injection more comfortable, less painful, and a better experience for the patient.
Myth #4: "Use of a vibrating distractor helps decrease injection pain."
Wiggling the patient's cheek or employing a vibrating device is not only a distraction technique, but helps blunt or block transmission of pain from receptors to the brain, much like rubbing a child's "boo-boo," which actually has a positive effect scientifically.
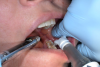
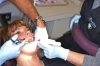
In 1965, Melzack and Wall described the Gate Control Theory, explaining a pain-modulating system in which a neural "pain gate" present in the spinal cord can open and close, thereby modulating the perception of pain.18 Some nerve fibers in the body are for pain, some are for touch; the dual transmissions of sensations race to the brain to be interpreted, each on its own track, but only one makes it there first. The smaller, unmyelinated Type A delta and Type C nerve fibers sense pain; those are what practitioners try to block with local anesthetics. Larger, myelinated Type A beta fibers are associated with touch, temperature, and pressure, and faster-moving sensations; these fibers are stimulated by wiggling the cheek. When pain and touch fibers are stimulated together, the gate will close and pain is not felt; touch is felt, rather than the pinch or prick of the needle (Figure 3), as the brain can perceive only one sensation at a time.
Three examples of vibrating devices are: first, a transcutaneous electronic nerve stimulation (TENS) unit which passes a high-frequency, low-voltage electric current between the two paths to activate the Type A beta fibers, sending signals to the brain that block or scramble normal pain signals; secondly, a vibrating device that snaps on to the barrel of an existing metal syringe; and third, a cordless, rechargeable handheld wand, featuring prongs with tips that vibrate; it also can be used as a retractor and intraoral light.
The latter two devices provide gentle, mild, vibrating sensations at the injection site to override the pain signals from the needle.
With the third device, vibration should be applied via light pressure for up to 15 seconds before the injection; the practitioner should inject as close to the prongs as possible, and then leave the vibration on for a few seconds after completing injection.
A study by Ching et al compared pain rating scale measurements from an exposure group, employing the third device, and a control group, in 36 adolescent patients age 10 to 17 in a split-mouth, paired-injections study using the Wong-Baker FACES Pain Rating Scale.19 The median difference between pain felt by the two groups was 2, with 17 of the patients on whom the practitioners used the third device reporting zero pain on injection, compared to only 3 by the control group.
In a study by Nasehi et al, researchers did two injections, one with the third vibrating device and one without, and they told patients where in their mouths the injection would be. They asked patients to rate how much pain the injection in the IA would cause using a Visual Analog Score. Anticipated pain without vibration was a mean of 5.37 and the actual was 5.26. With vibration, anticipated pain was 2.85, and actual was 1.73. Patients not only thought the injection would hurt less, but the actual pain was even less than anticipated. Researchers concluded that there was a significant reduction in the pain encountered during local anesthetic injection with the use of an intra-oral vibration device.20
A study by Shaefer et al used a "symptom severity index" and asked not only about the pain, but about the experience of the injection with the practitioner using the vibrating device. It showed use of the device was not only good for the pain, but also better for the entire patient experience.21
The practitioner holding the vibrating device may have to change his or her technique in terms of being unable to use fingers to retract or palpate structures such as the internal oblique ridge. As a workaround, have the dental assistant hold the pronged vibrating device; the practitioner can then use a finger to palpate the anatomy while the assistant puts the vibrating device as close to the injection point as possible (Figure 4 and Figure 5). Tips are single-use only; they snap on, and rip upon removal, preventing cross contamination.
Returning to the second vibrating device, it hooks onto the barrel of the syringe and is not separate from it as is the third device. Some literature shows a statistical difference in mean score of pain using this device, and some shows no statistically significant decrease in pain scores.22,23
Literature about anesthetic injection pain reduction in general shows that to be most effective, there needs to be two stimuli: the anesthetic injection needle and a vibrating device providing a non-painful stimulus. That is why the third device is more effective than the second, because it allows two separate stimuli rather than a vibrating device attached to, and operating as one with, a painful needle.
In any case, the efficacy of vibrating devices in reducing anesthetic injection pain is generally confirmed by the literature. Use of a vibrating device, particularly with the help of a dental assistant, therefore is recommended.
Myth #5: "I use small needles for my injections because they are less painful to the patient compared to larger needles. Small-gauge needles are less painful than large-gauge needles."
There is a longstanding belief in dentistry that using a smaller gauge needle will cause less discomfort to the patients. However, literature from so far back as the 1970s shows that patients are unable to differentiate among 23-, 25-, 27-, and 30-gauge needles,24 and there are no significant differences in the perception of pain produced by 25-, 27-, and 30-gauge needles during IA nerve blocks in adults.25
A study by Flanagan et al, using maxillary 25-, 27, and 30-gauge short needles and mandibular 25- and 27-gauge long needles, with pain rated on a zero-to-10 scale, concluded, "There is no statistically or clinically significant difference between perceived pain of injection based on the needle gauges commonly used in dentistry."26 The study involved 930 injections on 810 patients and evaluated 25-, 27-, and 30-gauge needles for maxillary injections and 25- and 27-gauge needles for mandibular injections. Patients were asked to rate the injection pain on a 0-10 scale where 0 is "no pain" and 10 is "the worst pain ever." Looking at the results and comparing the most common response by the patients shows that the 25- and 27-gauge needles were equivalent in patient discomfort, with the 30-gauge slightly better.
General recommendations include the use of 25- and 27-gauge needles, which are essentially indistinguishable to a patient, for IA block injection. For the practitioner these needle sizes offer a better chance of positive aspiration, less needle deflection when passing through tissue, and less chance of needle separation.27-30
A 30-gauge needle should be used with caution for a IA block, as they are short-around 22 mm. The depth of insertion for an IA block is 25 mm, so there is very little chance of achieving a positive aspiration. Instead, a 30-gauge needle is ideal for infiltration techniques.31-32
Although the verdict on Myth #5 is unclear, 25- and 27-gauge needles appear to be indicated for IA blocks, while a 30-gauge needle is indicated for infiltration.
Conclusion
While the conclusion concerning topicals decreasing injection pain is unclear, practitioners can achieve best results by taking into account patient experience, expectation, and age, and using topicals for the correct application time. It is confirmed that based on the type of solution employed by the practitioner, he or she can reduce injection pain by first providing a local anesthetic injection with prilocaine. While the benefit of warming cartridges is unclear, there is no downside and it may provide a better patient experience. The efficacy of vibrating devices in reducing anesthetic is generally confirmed. Lastly, although the verdict on smaller needles causing less pain is unclear, 25- and 27-gauge needles appear to be indicated for IA blocks, while a 30-gauge needle is indicated for infiltration.
About the Author
Jason H. Goodchild, DMD
Private Practice, Havertown, Pennsylvania, Vice President, Clinical Affairs, Premier Dental Products Co., Plymouth Meeting, Pennsylvania. Associate Clinical Professor, Department of Oral and Maxillofacial Surgery, Creighton University School of Dentistry, Omaha, Nebraska, Clinical Associate Professor, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
References
1. Meechan JG. Effective topical anesthetic agents and techniques. Dent Clin N Am. 2002;46:759-766.
2. Parirokh M, Sadeghi AS, Nakbaee N, et al. Effect of topical anesthesia on pain during infiltration injection and success of anesthesia for maxillary central incisors. J Endod. 2012;38:1553-1556.
3. Bhalla J, Meechan JG, Lawrence HP, et al. Effect of time on clinical efficacy of topical anesthesia. Anesth Prog.2009;56:36-41.
4. De Freiras GC, Pozzobon RT, Blaya DS, et al. Efficacy of benzocaine 20% topical anesthetic compared to placebo prior to administration of local anesthesia in the oral cavity: A randomized controlled trial. Anesth Prog. 2015;62(2):46-50.
5. Rehman N, Qazi SR. Efficacy of topical benzocaine in maxilla: A randomized controlled trial. Anesth Prog. 2019 Spring;66(1):
24-29.
6. Risk of serious and potentially fatal blood disorder prompts FDA action on oral over-the-counter benzocaine products used for teething and mouth pain and prescription local anesthetics. FDA Safety Announcement, 05-23-2018.
7. Topical Anesthetic-Induced Methemoglobinemia. PA PSRS Patient Saf Advis. 2005 Mar;2(1):17-8.
8. Goodchild JH, Donaldson M. Comparing the pH change of local anesthetic solution using two chairside buffering techniques. Compend Contin Educ Dent. 2016 May;37(5):e6-e12.
9. Kanaa MD, Meechan JG, Corbett IP, et al. Speed of injection influences efficacy of inferior alveolar nerve blocks: a double-blind randomized controlled trial in volunteers. J Endod.2006;32:919-23.
10. Kramp LF, Eleazer PD, Scheetz JP. Evaluation of prilocaine for the reduction of pain associated with transmucosal anesthetic administration. Anesth Prog. 1999 Spring;46(2):52-55.
11. Wahl MJ, Schmitt MM, Overton DA. Injection pain of prilocaine plain, mepivacaine plain, articaine with epinephrine, and lidocaine with epinephrine. Gen Dent. 2006 May-Jun;54(3):168-71.
12.. Wahl MJ, Schmitt MM, Overton DA, Gordon MK. Injection pain of bupivacaine with epinephrine vs. prilocaine plain. JADA.2002 Dec;133(12):1652-6.
13. Donaldson M, Goodchild JH. Pregnancy, breast-feeding and drugs used in dentistry. J Am Dent Assoc. 2012;143:858-871.
14. Dabarakis N, Tsirlis A, Parisis N, Tsoukalas D. The role of temperature in the action of mepivacaine. Anesth Prog. 2006 Fall;53(3):91-94.
15. Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011 Jul;58(1):86-98.
16. Lundbom JS, Tangen LF, Wågø KJ, et al. The influence of lidocaine temperature on pain during subcutaneous injection. J Plast Surg Hand Surg. 2017 Apr;51(2):118-121.
17. Aravena PC, Barrientos C, Troncoso C, et al. Effect of warming anesthetic on pain perception during dental injection; a split mouth-randomized clinical trial. Local Reg Anesth. 2018;11:9-13.
18. Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965 Nov. 19;150(3699); 971-9.
19. Ching D, Finkelman M, Loo C. Effect of the DentalVibe Injection System on pain during local anesthesia injections in adolescent patients. Pediatric Dentistry. 2014;36:51-5.
20. Nasehi A, Bhardwaj S, Kamath AT, et al. Clinical pain evaluation with intraoral vibration device during local anesthetic injections. J Clin Exp Dent. 2015 Feb; 7(1):e23-e27.
21. Shaefer JR, Lee, SJ, Anderson NK. A vibration device to control injection discomfort. Compend Contin Educ Dent. 2017 Jun;38(6):e5-e8.
22. Murray P, et al. Efficacy of a vibrating dental syringe attachment on pain levels. IADR 2003. Abstract #1177.
23. Saijo M, Ito E, Ichinohe T, et al. Lack of pain reduction by a vibrating local anesthetic attachment: A pilot study. Anesth Prog. 2005;52(2):62-64.
24. Howitt JW, Lowell C. Topical anesthetic effectiveness. An old and new product evaluated. N Y State Dent J. 1972 Nov.;38(9):549-550.
25. Fuller NP, Menke RA, Meyers WJ. Perception of pain to three different intraoral penetrations of needles. JADA.1979;99:822-824.
26. Flanagan T, Wahl MJ, Schmitt MM, Wahl, JA. Size doesn't matter; needle gauge and injection pain. Gen Dent. 2007 May-Jun.;55(3):216-7.
27. Malamed SF, Reed K, Poorsatter S. Breakage: incidence and prevention. Dent Clin N Am. 2010;54:745-56.
28. Robison SF, Mayhew RB, Cowan RD, Hawley RJ. Comparative study of deflection characteristics and fragility of 25-, 27-, and 30-gauge short dental needles. J Am Dent Assoc.1984;109:920-4.
29. Wong MK, Jacobsen PL. Reasons for local anesthesia failures. J Am Dent Assoc. 1992;123;69-73.
30. Meyer FU. Complications of local dental anesthesia and anatomical causes. Ann Anat. 1999;181:105-6.
31. Khoury J, Mihailidis S, Ghabriel M, Townsend G. Anatomical relationships within the human pterygomandibular space: Relevance to local anesthesia. Clin Anat.2010;23:936-44.
32. Ennes JP, de Medeiros RM. Localization of mandibular foramen and clinical implications. Int J Morphol. 2009;27(4):1305-1311.