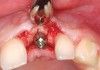
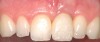
You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
One of the most demanding tasks for the dental clinician is single-tooth implant reconstruction in the esthetic zone. The esthetic and functional requirements for a successful long-term result often involve hard- and soft-tissue regeneration, and can challenge even the most experienced implant dentist.1
Immediately after tooth extraction, a number of biologic processes are initiated that lead to loss of alveolar bone and soft-tissue volume.2,3 An average of 40% to 60% of original height and width can be expected to be lost post-extraction, with most of that loss occurring within the first 2 years.4-6 Artzi et al7 demonstrated that the alveolar ridge resorbs up to 23% in the maxillary anterior region within 6 months of extraction, with an additional 11% resorption occurring in the subsequent 5 years.
Multiple site-preservation techniques can be used to minimize the potential morphologic changes that follow maxillary anterior tooth extraction. These include the use of bone allografts, xeno- grafts, autogenous bone, graft-stabilizing membranes, and autogenous and allogenic soft-tissue grafts and biologic modifiers. The extraction socket with an uncompromised alveolus and sufficient soft-tissue volume can be successfully treated with immediate implant placement.8 However, as the residual hard- and soft-tissue housing continues to be lost, both site-preservation and site-development procedures often become necessary to maintain existing tissue and regenerate lost tissue.
A pre-extraction clinical and radiographic evaluation does not always provide a comprehensive assessment of the existing hard- and soft-tissue volume. The periodontal attachment often masks the underlying hard-tissue architecture, and tooth removal can result in damage to the alveolus. Direct visualization of labial, palatal, and interproximal bone and a thorough evaluation of soft-tissue thickness are best accomplished after tooth extraction. This clinical assessment of the alveolus can reveal hard- and soft-tissue deficits ranging in severity from mild to severe. The therapeutic goal of hard- and soft-tissue augmentation is to create an optimized architecture for predictable esthetic and functional implant rehabilitation. This article reviews criteria for the evaluation of both pre- and post-extraction tissue deficits, and presents appropriate regenerative and augmentation strategies for optimal implant site development.
Rationale for Alveolar Ridge Preservation
Tooth loss inevitably results in alveolar ridge resorption, as evidenced by a number of studies.9,10 In a 10-case study, Lekovic et al11 compared the outcome of alveolar ridge preservation after extractions using absorbable barrier membranes alone. At 6 months, significantly less crestal bone loss (-0.38 mm vs -1.50 mm), more internal socket fill (-5.81 mm vs -3.94 mm), and less horizontal ridge resorption (-1.31 mm vs -4.56 mm) were found in the membrane group than in the control group.
Histologic investigation in animals12 and humans13,14 have described the healing of extraction sockets. Cephalometric analyses9,15,16 and measurements on study models17,18 have shown gross morphologic changes of the alveolar processes after tooth loss. Pietrokovski and Massler17 demonstrated resorption of the alveolar process after tooth extraction in the maxilla or mandible to be significantly larger at the buccal aspect as compared with the remaining alveolar process.
The maximum loss of tissue contour occurs during the first few months after tooth extraction. Schropp et al19 assessed bone formation in the alveolus and contour changes of the alveolar process after tooth extraction. They followed 46 patients over a 12-month period by means of measurements on study casts, linear radiographic analyses, and subtraction radiography. This prospective clinical trial demonstrated that major changes of an extraction site take place during the 12 months after tooth extraction. The width of the alveolar ridge was reduced by 50% during the observation, which is in agreement with earlier studies.17,18,20 Approximately two thirds of this reduction occurred within the first 3 months after tooth extraction, also corresponding to earlier findings.18,20,21 Bone formation in the alveoli and loss of height of the alveolar bone crest also occurred simultaneously during the first 3 months. Formation of bone continued during the next 3 months, and from 6 to 12 months a portion of new bone underwent remodeling. The amount of alveolar bone loss was almost unchanged from 3 to 12 months. Additionally, the loss of crestal bone height mainly occurred within the 3-month period after tooth extraction, with reorganization of the lamina dura occurring during the entire healing period.
In regard to linear measurements on radiographs, Schropp and colleagues19 showed that the level of new bone generated in the extraction sockets never reached the levels of bone attachment at the tooth surfaces mesial and distal to the extraction sites. Other observations suggested that the bone level at the extraction sites, rather than the bone level of the adjacent teeth, dictates the level to which the bone crest heals after extraction.
Tarnow et al,22 in a clinical study of the natural dentition, determined that the presence or absence of interproximal papilla fill was inversely related to the distance from the base of the contact area to the underlying crest of bone. At a distance of 5 mm or less, the papilla fill was present almost 100% of the time. When the distance measured 6 mm, papilla fill was present 56% of the time and, at a distance of 7 mm or more, papilla fill was present in only 27% of the sites examined. Bone density and radiographs were used predominantly to arrive at these measurements. Salama et al23 have suggested that a similar relationship exists in implant therapy; height, width, and depth of peri-implant papillae contours may be affected by this correlation. The authors emphasized that the most successful and predictable esthetic results can be accomplished only when underlying labial and interproximal osseous support is provided therapeutically for the desired soft-tissue contours. In another study, Salama et al24 presented a classification of interproximal height of bone as defined from the cementoenamel junction and future contact points. They demonstrated the importance of anatomic interproximal height of bone being dominant over implant interproximal height of bone in determining papilla length. This classification further demonstrates the importance of preserving interproximal bone, especially at the time of extraction.
Pre-extraction Assessment
A number of diagnostic parameters that can affect the treatment outcome must be evaluated before extraction. These include a detailed medical and dental history, a comprehensive esthetic and peri- odontal evaluation, and a thorough soft- and hard-tissue assessment of the existing tooth, the adjacent teeth, and the extraction socket.25
Medical History
A thorough patient medical assessment plays a major role in contributing to the overall success of dental implant reconstruction. The medical questionnaire, along with the patient interview, can provide important insight into the patient’s medical status and identify potential systemic problems that may com- promise wound healing. A number of risk factors can impair soft- and hard-tissue regeneration, including smoking, drug and alcohol abuse, impaired renal or hepatic function, long-term corticosteroid use, uncontrolled diabetes, and any general systemic disease that can compromise bone metabolism. The implant dentist must be able to evaluate each systemic disease and condition and understand its impact on patient selection and management. Systemic diseases can manifest a significant number of wound healing problems, depending on the disease and its severity. Only some conditions are absolute contraindications for regenerative implant surgery; a number of metabolic disorders become contraindicated when the disease process is out of control. Examples of absolute contraindications include leukemia, chronic alcoholism, diabetes mellitus with vascular complications, severe renal failure, and unstable angina. An alteration of surgical protocol may be necessary when dealing with some medically compromised patients and treatment outcomes are not expected to be optimal.
Dental Etiology
A comprehensive evaluation of the patient’s overall dental health is essential to predictable single-tooth implant rehabilitation in the esthetic zone. A detailed history of regional and tooth-specific therapeutic interventions, including any previous restorative, periodontal, and endodontic treatment, is critical to the interpretation of soft- and hard-tissue health and treatment planning for implant site regeneration. Identification and management of contributory dental etiologic factors, with careful assessment of the occlusal stability of the remaining dentition, is essential for esthetic implant reconstruction.
Esthetic Assessment
A comprehensive systematic dentofacial and dentoalveolar esthetic evaluation must be performed before tooth extraction. This examination should begin with the evaluation of facial and dental symmetry. Assessment of the upper lip line and labial form, along with their relationships to the underlying dentoalveolar complex, should be performed at rest and with an exaggerated smile posture. Incisal and occlusal planes, tooth proportions and their relationships, gingival plane, and gingival outline also should be included.
The dentoalveolar evaluation will provide for precision treatment planning and dictate the use of any appropriate adjunctive procedures, such as orthodontic therapy.26 Orthodontic extrusion, a form of distraction osteogenesis, can be used to reposition hard and soft tissues that can contribute to an optimal esthetic result. Orthodontic therapy also can reposition dentition to create optimal interdental and crown height space before im- plant placement. Current accepted guidelines suggest a minimum of 1.5 mm to 2 mm of space between an implant and the adjacent teeth, and 3 mm between two adjacent implants to maintain interproximal bone and soft tissue.27,28
Periodontal Assessment
A thorough periodontal examination before tooth extraction is critical to overall management of the extraction site. This examination should include appropriate two-dimensional and three-dimensional radiographic imaging. The periodontal examination should assess the gingival tissue status, probed attachment levels, recessions, mucogingival junction, mobility, and periodontal biotype, as well as the presence of plaque and related gingival alveolar pathology.
Periodontal Biotype
A key factor in achieving an optimal esthetic result with single-tooth implant placement in the esthetic zone is the patient’s periodontal biotype. Olsson and Lindhe29 described two distinct periodontal biotypes and correlated them with specific tooth shapes, soft-tissue profile, and underlying osseous architecture. Implant dentists must understand how the two periodontal biotypes respond to both surgical and restorative therapy.
The thin, scalloped periodontium is characterized by thin, friable gingivae, long and pointy interproximal papillae, and minimal amounts of attached keratinized tissue. Also present is a thin underlying alveolar bone, which is typically fenestrated or dehisced.29 After surgical intervention with this biotype, dentists should expect some degree of soft-tissue recession in addition to alveolar bone resorption.30 Every effort must be made to preserve the existing hard- and soft-tissue anatomy by using minimally invasive surgical and restorative procedures. In contrast, the thick, flat periodontium is associated with relatively flat soft-tissue and osseous architecture. This bio- type is usually seen with a square and wide tooth form. In addition, it is characterized by thick, fibrotic gingiva that is resistant to recession, and short and flat interproximal papillae with wide zones of attached keratinized tissues. The accompanying thick underlying alveolar bone is typically resistant to resorption; however, the thick fibrotic soft tissues are predisposed to forming notches and scars that may require multiple revisions.29
In clinical dental practice, the majority of patients present with one of the two aforementioned periodontal biotypes. However, a moderate hybrid biotype, which possesses features of both thin, scalloped and thick, flat soft-tissue architectures, also can be present. The surgical management of this biotype is similar to that of the thin scalloped biotype.
Atraumatic Tooth Extraction
Tooth extraction should be performed using a relatively atraumatic and flapless approach, ideally involving the use of a periotome, rotary burs, and extraction forceps. Radiographic imaging of the surgical site should be used to identify root morphology, surrounding anatomic structures, and bony pathology. A sulcular incision should be performed to initiate sep- aration of epithelial and connective-tissue attachments to the tooth surface. De-epithelialization of the sulcular tissue should be accomplished using either a blade or diamond bur to provide a vascular supply for any necessary soft-tissue augmentation. The use of sharp surgical blades will minimize trauma and loss of the gingival tissues.
Straight-handle periotomes should be used to luxate the tooth within the depth of the gingival sulcus, which will result in circumferential separation of the gingival attachment. Using continued apical pressure, the instrument should be inserted into the periodontal ligament space along the root surfaces to sever the periodontal ligament directly below the alveolar crest. This process should be continued until the periotome penetrates to a depth sufficient to initiate adequate tooth mobility for simple forceps extraction. Use of the periotome should be limited to interproximal and palatal areas. Preservation of the labial plate is critical to achieve an optimal esthetic result. Conventional rotary instrumentation as well as piezosurgery burs can be used as needed for ankylosed teeth and fractured subgingival roots.
After tooth removal, the alveolar socket should be debrided of all granulation tissue. Bleeding should be stimulated from the osseous walls through the use of rotary instruments or curettes. This protocol has been shown to trigger the regional acceleratory phenomenon, which stimulates new bone formation and graft incorporation.31
Next, the extraction socket should be evaluated visually and tactilely. A periodontal probe can be used to sound the labial, palatal, and interproximal bone morphology. Special attention should be given to direct visualization of the labial plate’s integrity. This examination can identify fenestration as well as dehiscence defects. Labial and palatal plate thickness also should be examined. Ideally, a minimum of 2+ mm of labial plate thickness is adequate for implant support without esthetic or functional compromise.32 A thin labial plate (< 2 mm) can lead to further bone loss, often resulting in partial or complete cortical plate compromise. Vertical bone loss also can be present with the labial and palatal plates, as well as with the interproximal bone.
Depending on the degree of hard- and soft-tissue loss, a treatment plan can be formulated that includes both hard- and soft-tissue augmentation as well as regenerative procedures. This author has found that the use of alveolar socket grafting with mineralized irradiated allograft, mandibular block autografts, free gingival grafts, and connective tissue grafts can result in predictable esthetic and functional reconstruction of esthetic zone single-tooth extraction sites.
Clinical Extraction Site Management
Extraction site management is based on the extent of alveolar bony tissue loss as categorized by this author into mild, moderate, and severe vertical labial plate compromise. A minimum labial plate thick- ness of 2 mm is recommended for an optimal esthetic and functional result with all cases, regardless of category. Periodontal biotype will impact clinical management of the specific osseous defect. In addition, class I interproximal height of bone is required for predictable regeneration of the alveolus.24
Mild tissue degradation in the presence of a thick, flat periodontal biotype is characterized by less than 3 mm of vertical labial plate loss. Management consists of socket grafting with mineralized bone allograft and a free gingival graft.33,34 A 4-month healing period is necessary before implant placement. This protocol is modified in the presence of a thin, highly scalloped biotype where a connective tissue graft35 is recommended in place of a free gingival graft to help increase soft-tissue volume. If the labial plate is less than 2 mm, then a bone xenograft is used as a veneer graft.
Moderate tissue degradation in the presence of a thick or thin biotype is characterized by labial plate loss of 3 mm to 6 mm. Surgical management includes socket grafting with mineralized bone allograft and use of a connective tissue graft. This may result in sufficient hard- and soft-tissue volume to allow implant placement on healing. Reevaluation at 6 months will direct the need for additional hard- or soft-tissue augmentation or confirm acceptable bone architecture for implant placement. Additional procedures include guided bone regeneration (GBR), veneer xenograft, and connective tissue graft.
Severe tissue degradation is characterized by 7 mm or greater labial plate loss in the presence of either periodontal biotype. Management includes socket grafting with mineralized bone allograft and the use of a connective tissue graft. These defects require a healing time of 7 to 8 months and often require additional augmentation that may include GBR, autogenous mandibular bone blocks, and additional connective tissue grafts (Table 1).
| Defect | Labial Plate Loss | Treatment | Time to Implant Placement |
| Mild | < 3 mm | MBA, FGG, veneer xenograft | 4+ months |
| Moderate | 3 mm to 6 mm | MBA, CTG; re-evaluate; GBR, veneer xenograft, CTG | 6 months |
| Severe | ≥ 7 mm | MBA, CTG; re-evaluate; MBG, CTG | 7 to 10 months |
The case presentations that follow exhibit a range of post-extraction labial plate compromise from mild to severe. The treatment plans for extraction management are presented and include examples of the author’s recommendations as described in this article.
Case 1: Treatment Strategy for Mild Tissue Degradation (Labial Plate Loss)
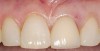
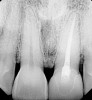
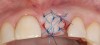
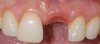
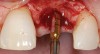
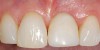
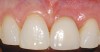
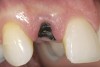
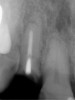
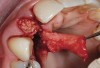
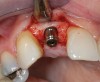
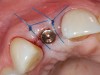
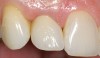
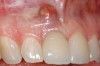
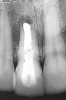
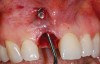
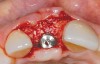
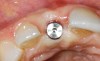
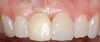
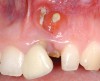
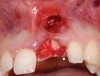
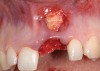
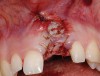
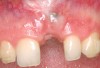
A 59-year-old woman presented with a recent history of trauma to her pre-maxilla, resulting in vertical root fracture of the maxillary left central incisor (Figure 1 and Figure 2). Examination revealed a mobile crown, moderate flat periodontal biotype, gingival margin of the left central incisor 1 mm coronal to that of the adjacent central incisor, central incisor tooth width discrepancy, and a medium smile line. The treatment plan included flapless extraction of the fractured tooth with the potential for simultaneous socket grafting using mineralized bone allograft (MinerOss®, BioHorizons, Inc, Birmingham, AL) and a free gingival graft (Figure 3) harvested from the left palate. Post-extraction examination revealed vertical labial plate loss of 2 mm and class I interproximal height of bone; therefore, all three procedures were performed. Four months postsurgery (Figure 4), a mid-crestal and sulcular incision without release was made to allow for crestal plasty and core biopsy (Figure 5). Histomorphometry revealed 87% vital bone with minimal evidence of residual allograft (Figure 6). This finding was consistent with type II bone density and allowed for non-submerged implant placement. The labial plate was completely regenerated. A provisional crown was placed and contoured to groom the soft tissue for 3 months (Figure 7), followed by final crown fabrication (Figure 8).
Case 2: Treatment Strategy for Moderate Tissue Degradation (Labial Plate Loss)
A 54-year-old woman was referred for treatment of a fractured maxillary right lateral incisor, secondary to recurrent subgingival decay. Clinical and radiographic examination revealed a high smile line; a thin, highly scalloped biotype; class II deep bite; a thin labial plate (< 2mm) with 4 mm of vertical bone loss; and class I interproximal height of bone24 (Figure 9 and Figure 10).
Treatment consisted of flapless extraction, socket grafting using mineralized bone allograft (Puros®, Zimmer Dental Inc, Carlsbad, CA), and a pedicled connective tissue graft36,37(Figure 11). Five months post-surgery, reentry was accomplished via a mid-crestal and labial sulcular incision. A crestal plasty was per- formed before implant placement into type II bone (Figure 12 and Figure 13). An immediate provisional was used for 3 months (Figure 14) before final crown fabrication (Figure 15).
Case 3: Treatment Strategy for Severe Tissue Degradation (Labial Plate Loss)
A 39-year-old woman presented with a failing endodontically treated maxillary right central incisor. Clinical and radiographic examination revealed a thick, flat periodontal biotype, high smile line, and a fistulous tract of the labial vestibule opposite the incisor (Figure 16 and Figure 17). Also noted post-extraction was a thin (< 2 mm) labial plate with 8 mm of vertical bone loss (Figure 18). Treatment consisted of a conservative flapless extraction with placement of a free connective tissue graft (Figure 19) sutured through the socket, along with placement of a bone mineralized allograft (MinerOss) (Figure 20 and Figure 21). Five months post-extraction, a root-form implant was placed, nonsubmerged, along with a bovine bone xenograft as a veneer into a sub-periosteal labial pouch (Figure 22 through Figure 24). A provisional crown was placed 3 months later, allowing for adequate soft-tissue grooming (Figure 25).
Case 4: Treatment Strategy for Severe Tissue Degradation (Labial Plate Loss)
A 26-year-old woman was referred for evaluation and treatment of a fractured maxillary left central incisor. Clinical and radiographic examination revealed a fractured endodontically treated tooth with a fistulous tract in the labial vestibule (Figure 26). Also noted were a flat, thick periodontal biotype, a medium smile line, and a complete loss of labial plate. Atraumatic tooth removal using a periotome and thin-beaked forceps revealed the extent of soft- and hard-tissue loss (Figure 27). Initial treatment consisted of a mineralized bone allograft (MinerOss), along with soft-tissue grafting (connective tissue graft and free gingival graft) (Figure 28 and Figure 29). Reevaluation 9 months later revealed regeneration of a thicker labial plate that was still deficient in both width and height for conventional root-form implant placement. A mandibular block graft harvested from the symphysis was used as Phase II treatment (Figure 30). After 4 months, a root-form implant was placed in a nonsubmerged mode (Figure 31) in preparation for provisional crown fabrication (Figure 32).
Discussion
Tooth extraction is known to result in alveolar bone loss secondary to atrophy of the edentulous ridge.2-6 A number of procedures have been proposed in an attempt to preserve and regenerate hard- and soft-tissue alveolar anatomy. Soft-tissue procedures include the use of connective tissue grafts, both free and pedicled, as well as acellular dermis matrix.38 A commonly used hard-tissue procedure is immediate socket grafting with a variety of materials, including autogenous bone, demineralized freeze-dried bone allograft,39 mineralized freeze-dried bone allograft,40 bovine hydroxylapatite,41 and alloplasts.42
This author’s preference is to use human mineralized and irradiated allograft (MinerOss) for alveolar socket preservation and regeneration. This allograft has been found to predictably form type II bone while providing an excellent bony matrix with load-bearing capabilities. Core biopsies of multiple specimens over the past 8 years have shown consistent lamellar bone formation within 4 months after grafting and the allograft appears to be completely resorbed and replaced by host bone within 4 to 5 months. This graft material consists of a combination of cancellous and cortical bone particles that range in particle size from 0.6 mm to 1.2 mm. Another human mineralized irradiated allograft (Puros) also can be used with equally predictable results. This author has used cancellous bone only within the particle range of 1 mm to 2 mm. This allograft material, both cancellous and cortical, is processed through a unique solvent-preserved process for tissue preservation and viral inactivation, which differs from the standard cryo-preserved process.
Although complete regeneration of severe labial plate defects is not commonly accomplished, a secondary GBR technique can be performed with predictable results, avoiding the need for more invasive procedures, such as autogenous mandibular block grafts. In cases with thin labial plates, a slow resorbing material, such as bovine hydroxyapatite (Bio-Oss®, Osteohealth, Shirley, NY) can be used for augmentation that satisfies both the esthetic and functional demands of labial plate morphology.
Conclusion
With the profession’s increasing focus on conservative esthetic dentistry, single-tooth implant reconstruction has become the therapy of choice for tooth replacement in the presence of adjacent healthy natural dentition and dentoalveolar tissues. In contrast to the posterior single-tooth implant, the esthetic zone single- tooth implant can provide a myriad of technical, surgical, and regenerative challenges for the surgeon directly proportional to localized clinical pathology and remaining hard-and soft-tissue extraction socket architecture. In an ongoing effort to restore and mimic nature, this author has found that the consistent application of the discussed sequential management protocol for anterior single-tooth extraction sites can result in predictable tissue regeneration appropriate to the demands of an optimized esthetic zone implant rehabilitation.
Disclosure
The author has received honoraria and is a current consultant for BioHorizons, Inc.
References
1. Garber D. The esthetic dental implant: letting restoration be the guide. J Am Dent Assoc. 1995:126(3):319-325.
2. Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites: an experimental study in dogs. J Clin Periodontol 2003;30(9):809-818.
3. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 2005;32(2):212-218.
4. Werbitt MJ, Goldberg PV. Immediate implantation. Preservation of bone volume and osseous regeneration [in French]. J Parodontol. 1991;10(2):157-166.
5. Werbitt MJ, Goldberg PV. The immediate implant: bone preservation and bone regeneration. Int J Periodontics Restorative Dent. 1992;12(3):206-217.
6. Polizzi G, Grunder U, Goené R, et al. Immediate and delayed implant placement into extraction sockets: a 5-year report. Clin Implant Dent Relat Res. 2000;2(2):93-99.
7. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000;71(6):1015-1023.
8. Chen ST, Wilson TG Jr, Hämmerle CH. Immediate or early placement of implants following tooth extraction: review of biologic basis, clinical procedures, and outcomes. Int J Oral Maxillofac Implants. 2004;19 (Suppl):12-25.
9. Atwood DA, Coy WA. Clinical, cephalometric, and densitometric study of reduction of residual ridges. J Prosthet Dent. 1971;26 (3):280-295.
10. Atwood DA. Reduction of residual ridges: a major oral disease entity. J Prosthet Dent. 1971;26(3):266-279.
11. Lekovic V, Kenney EB, Weinlaender M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997; 68(6):563-570.
12. Huebsch RF, Hansen LS. A histopathologic study of extraction wounds in dogs. Oral Surg Oral Med Oral Pathol 1969;28(2):187-196.
13. Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:46-48.
14. Boyne PJ. Osseous repair of the postextraction alveolus in man. Oral Surg Oral Med Oral Pathol. 1966;21(6):805-813.
15. Carlsson GE, Bergman B, Hedegård B. Changes in contour of the maxillary alveolar process under immediate dentures. A longitudinal clinical and x-ray cephalometric study covering 5 years. Acta Odontol Scand. 1967;25(1):45-75.
16. Carlsson GE, Persson G. Morphologic changes of the mandible after extraction and wearing of dentures. A longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Revy. 1967;18(1):27-54.
17. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.
18. Johnson K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust Dent J. 1969;14(4): 241-244.
19. Schropp L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
20. Johnson K. A study of the dimensional changes occurring in the maxilla following tooth extraction- Part 1. Normal healing. Aust Dent J. 1963;8:428-433.
21. Lam RV. Contour changes of the alveolar processes following extractions. J Prosthet Dent. 1960;10(1):25-32.
22. Tarnow DP, Magner AW, Fletcher P. The effect of distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J Periodontol 1992;63(12):995-996.
23. Salama H, Salama M, Kelly J. The orthodontic-periodontal connection in implant site development. Pract Periodontics Aesthet Dent. 1996;8(9):923-932.
24. Salama H, Salama MA, Garber D, et al. The interproximal height of bone: a guidepost to predictable aesthetic strategies and soft tissue contours in anterior tooth replacement. Pract Periodontics Aesthet Dent. 1998;10(9):1131-1141.
25. Caplanis N, Lozada JL, Kan J. Extraction defect assessment, classification, and management. J Calif Dent Assoc. 2005; 33(11):853-863.
26. Salama H, Salama M. The role of orthodontic extrusive remodeling in the enhancement of soft and hard tissue profiles prior to implant placement: a systematic approach to the management of extraction defects. Int J Periodontics Restorative Dent. 1993; 13(4):312-333.
27. Esposito M, Ekestubbe A, Gröndahl K. Radiological evaluation of marginal bone loss at tooth surfaces facing single Brånemark implants. Clin Oral Implants Res. 1993; 4(3): 151-157.
28. Tarnow D, Elian N, Fletcher P, et al. Vertical distance from the crest of bone to the height of the interproximal papilla between adjacent implants. J Periodontol. 2003; 74(12):1785-1788.
29. Olsson M, Lindhe J. Periodontal characteristics in individuals with varying form of the upper central incisors. J Clin Periodontol. 1991;18(1):78-82.
30. Kois JC. Predictable single-tooth peri-implant esthetics: five diagnostic keys. Compend Contin Educ Dent. 2004;25(11):895-900.
31. Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Rel Res. 1989;(248):283-293.
32. Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005; 25(2):113-119.
33. Landsberg CJ, Bichacho N. A modified surgical/prosthetic approach for optimal single implant supported crown. Part I—the socket seal surgery. Pract Periodontics Aesthet Dent. 1994;6(2):11-19.
34. Tal H. Autogenous masticatory mucosal grafts in extraction socket seal procedures: a comparison between sockets grafted with demineralized freeze-dried bone and deproteinized bovine bone mineral. Clin Oral Implants Res. 1999;10(4):289-296.
35. Kan JY, Rungcharassaeng K, Lozada JL. Bilaminar subepithelial connective tissue grafts for immediate implant placement and provisionalization in the esthetic zone. J Calif Dent Assoc. 2005;33(11):865-871.
36. Nemcovsky CE, Artzi Z. Split palatal flap. A surgical approach for primary soft tissue healing in ridge augmentation procedures: technique and clinical results. Int J Periodontics Restorative Dent 1999;19(2):175-181.
37. Nemcovsky CE, Artzi A, Moses O. Rotated split palatal flap for soft tissue primary coverage over extraction sites with immediate implant placement. Description of the surgical procedure and clinical results. J Periodontol 1999;70(8):926-934.
38. Callan DP, Silverstein LH. Use of acellular dermal matrix for increasing keratinized tissue around teeth and implants. Pract Periodontics Aesthet Dent 1998;10(6):731-734.
39. Becker W, Becker BE, Caffesse R. A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets [published erratum appears in: J Periodontol. 1995; 66(4):309]. J Periodontol. 1994;65(12): 1128-1133.
40. Feuille F, Knapp Cl, Brunsvold MA, et al. Clinical and histologic evaluation of bone-replacement grafts in the treatment of localized alveolar ridge defects. Part 1: mineralized freeze-dried bone allograft. Int J Periodontics Restorative Dent. 2003;23(1):29-35.
41. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histmorphometric evaluations at 9 months. J Periodontol. 2000;71(6):1015-1023.
42. Froum S, Orlowski W. Ridge preservation utilizing an alloplast prior to implant placement—clinical and histological case reports. Pract Periodontics Aesthet Dent. 2000; 12(4):393-404.Michael A. Pikos, DDS
Michael A. Pikos, DDS
Pikos Implant Institute
Palm Harbor, Florida
Adjunct Assistant Professor of Surgery
Department of Oral & Maxillofacial Surgery
College of Dentistry, The Ohio State University
Columbus, Ohio