You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Applications for ceramics in dentistry became increasingly popular in the 18th century, largely due to the esthetic characteristics of the material compared to other tooth substitutes.1 Alexis Duchateau, a Parisian apothecary, integrated ceramics into dentistry when he created a complete set of dentures using porcelain ceramic material.2 Later, in 1903, Charles Land further advanced dental ceramics by developing all-ceramic inlays, onlays, and crown restorations using fired porcelains,3,4 innovations that led to the creation of porcelain jacket crowns.5
Since then, dental ceramics have evolved with modifications to their chemical composition, esthetic properties, manufacturing processes, packaging, and indications. Highly esthetic and biocompatible results were achieved with early versions of dental ceramics, but the material’s weakness in tensile and shear stresses necessitated development of ceramic materials with greater strength and durability,6-8 especially when thicker restorations are necessary and/or cementing mainly to dentin is required.
Along with CAD/CAM technology, today’s pressable and millable materials enable fabrication of stronger and more minimally invasive ceramic restorations that are also esthetic.9,10 This facilitates selection of the optimal metal-free ceramic material based on the specific treatment, since newer ceramic materials are stronger, easier to use, and versatile.
However, selecting the appropriate ceramic material also depends upon technique.6,11,12 Unfortunately, contradicting information has created confusion about which ceramic materials and restorative techniques are suitable for specific clinical situations.13 Understanding the classifications, composition, and characteristics of today’s all-ceramic materials allows dentists and laboratory technicians to determine the ideal material for a given treatment.
Composition, Characteristics, and Classification
Ceramics are inorganic, nonmetallic solids produced by the heating at high temperatures and subsequent cooling of raw compounds such as nitrides, carbides, metal oxides, and borides, as well as mixtures of these materials. Therefore, a material labeled as ceramic is in fact not ceramic by definition if it is created by another processing technique or has organic components.
Ceramic materials may contain a crystalline or partly crystalline structure, or they may be amorphous (eg, a glass). Since most dental ceramics have at least some crystalline component, some authors limit the definition of ceramics to inorganic crystalline-containing materials, rather than including non-crystalline glasses, even though glasses are ceramics.14,15
Understandably, dental ceramics are generally categorized by their microstructure,9 which facilitates scientific understanding of the structural and chemical nature of dental ceramics but does little to aid dentists or ceramists in selecting the appropriate material for a given clinical situation. The manner in which a ceramic is processed greatly influences its mechanical behavior and, therefore, its clinical behavior. Therefore, classifying dental ceramics based on their composition and how they are processed can better provide clear clinical parameters for evaluating and appropriately choosing the most conservative ceramic for each clinical situation.16 The categories below are presented from most conservative to least conservative in terms of healthy tooth structure preservation. The following is an update to a previously published classification system that takes into account increased clinical documentation of the success of newer glass ceramics, and introduces some new materials.16
CL-I (Powder/Liquid)
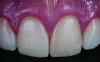
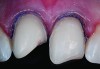
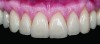
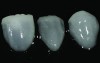
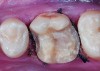
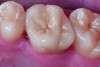
Class I (CL-I) powder and liquid porcelains are created from materials primarily containing silicon dioxide and possess a glassy matrix and varying amounts of a crystalline phase within the glassy matrix (eg, Creation Porcelain, Jensen Dental, www.jensendental.com; Ceramco 3, DENTSPLY International, www.dentsply.com; EX-3, Kuraray Noritake Dental, Inc, www.kuraraynoritake.com). The CL-1 group includes feldspathic porcelains, referred to as such because they were originally—and some continue to be—made from naturally occurring feldspars (ie, aluminosilicates composed of assorted quantities of potassium, sodium, barium, or calcium).9,17 Several feldspathic material options are available on the market today (eg, VITA VM 13, VITA Zahnfabrik, www.vita-zahnfabrik.com; Vintage Halo, Shofu, www.shofu.com) (Figure 1 through Figure 3).
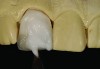
CL-I materials are fabricated by hand (Figure 4); they are the most conservative and generally the most translucent ceramic materials, but they are also the weakest.9,10,18 The material’s high translucency and esthetics create the illusion of natural teeth.9 Powder/liquid porcelain materials are ideal for cases in which significant enamel remains and/or there is healthy tooth structure on the teeth (ie, 50% or more remaining enamel on the tooth, 50% or more of the bonded substrate is enamel, and 70% or more of the margin is in the enamel). Feldspathic porcelain restorations that are bonded to primarily enamel substrates have proven to be highly successful long term.19
Powder/liquid porcelains demonstrate high esthetics and workability, and because they can be layered very thinly and placed directly on the enamel, they are considered the most conservative of the metal-free ceramic classes.10 CL-I porcelains require a thickness of 0.2 mm to 0.3 mm for each shade change.20,21
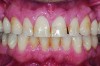
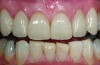
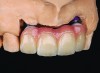
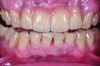
This class of materials is generally indicated for anterior restorations, but can also be used for the occasional bicuspid and rare molar, providing all parameters are at a very low risk level (Figure 5 and Figure 6).
CL-II (Glass Ceramics)
The composition of CL-II ceramics is similar to CL-I porcelain in that both possess a glassy matrix, but the two classes vary in their glass-crystalline ratios and crystal types. In CL-II materials, crystal types can either be added to the glass or grown into the glassy matrix. CL-II ceramics also differ from CL-I porcelains in manufacturing, as they are formed into dense industrial blocks for pressing and machining. Based on their crystal type and documented clinical behavior, CL-II pressed and machined glass ceramics can be further subdivided into two distinct groups.
CL-IIa
Materials in this subdivision contain low-to-moderate (<50%) leucite-containing feldspathic glass. Such materials (eg, IPS Empress® CAD, Ivoclar Vivadent, www.ivoclarvivadent.com; Authentic® Jenson Dental; VITABLOCS® Mark II, VITA Zahnfabrik) contain less than 50% crystalline and perform more like a glass, which requires bonding.
Like all CL-II materials, which have come to be known as glass ceramics, CL-IIa materials can be used for the same indications as CL-I materials—including anterior teeth, bicuspids, and rarely molars. Additionally, they have documented long-term clinical success in higher stress situations or when more dentin is exposed. They may be highly translucent, but traditionally they have required slightly thicker dimensions for workability and esthetics/shade matching (ie, minimum working thickness of 0.8 mm if layered with a veneering porcelain) (Figure 7 and Figure 8).20,21
Materials in this subcategory demonstrate increased material strength, primarily due to the processing technique of using a dense, industrial-made block, and possibly due to the leucite and its ability to alter the coefficient of thermal expansion, inhibiting crack propagation. These dense glass- and leucite-containing materials are indicated for thicker veneers, anterior crowns, and posterior inlays and onlays, but only when a long-term bond and seal can be maintained.
CL-IIb
This is a new subcategory that includes moderate-to-high (ie, >50%) crystalline-containing glass or glass ceramics. The material’s microstructure consists of a glass matrix surrounding a second phase of individual crystals. It originates as homogeneous glass, after which a secondary treatment nucleates and grows crystals, a process that imparts improved mechanical and physical properties by maximizing the presence of crystals and the generation of compression stress around the crystals.
An example of this material subcategory is lithium disilicate (eg, IPS e.max®, Ivoclar Vivadent), a glass ceramic material composed of silica, lithium dioxide, alumina, potassium oxide, and phosphorous pentoxide. After the crystalline component has reached optimal growth through the manufacturing process, it is pulverized into powder and processed through a variety of different techniques.22 Lithium disilicate is indicated for the same clinical situations as other glass ceramics; however, when fabricated to a full-contour, monolithic restoration and seated with resin cement, it is also appropriate for higher stress situations, such as those requiring full crowns, even on molars (Figure 9 through Figure 11).
New additions to the category are zirconia-reinforced lithium silicates (ZLSs) (eg, VITA Suprinity®, Figure 12; CELTRA™ Duo, DENTSPLY). ZLS materials comprise a lithium silicate glass ceramic that is strengthened with approximately 10% zirconia crystals. Although these materials are new to the market as of press time, initial in vitro testing shows they have excellent optics and physical properties similar to lithium disilicates. Only lithium disilicates have long-term clinical data to support their use as single restorations anywhere in the mouth, however.
Restorations fabricated from this material subcategory demonstrate high strength, fracture resistance, and natural-looking esthetics,23 yielding a versatile and strong alternative for a wider variety of indications. They are indicated when higher risks are involved (eg, less than 50% enamel remains on the tooth, less than 50% of the bonded substrate is enamel, and/or when 30% or more of the margin is in dentin).
Due to the material’s glass properties, adhesive bonding is recommended. However, bonding to dentin results in less predictable restorations due to dentin’s flexibility; restorations bonded to enamel are much more predictable, given enamel’s significant stiffness compared to dentin.19
CL-III (High-Strength Crystalline)
CL-III materials are high-strength crystalline ceramics with minimal or no crystalline phase, and are also produced through industrial processes. They differ from glass or glass ceramics based on the manner in which a sintered crystalline matrix of high-modulus material (85% to 100% of the volume) creates a junction with the particles in the crystalline phase.
CL-IIIa
CL-IIIa materials are manufactured by creating a porous matrix that is formed into a block, and then final processed to shape using CAD/CAM technology, after which a second-phase material melts and fills the pores within the material. Lanthanum aluminosilicate glass is drawn in either a liquid or molten glass form into all of the pores via capillary action, creating a dense and interpenetrating material from the internal to external surfaces. The final material is an 85% crystalline mesh infused with a small amount of glass. This material is disappearing from the marketplace and being replaced entirely by 100% polycrystalline ceramics.
CL-IIIb
CL-IIIb high-strength 100% crystalline ceramics initially were alumina-based materials (Procera®, Nobel Biocare, www.nobelbiocare.com), and more recently zirconia-based materials (eg. LAVA™, 3M ESPE, www.3mespe.com; Prettau®, Zirkonzahn, www.zirkonzahn.com). Alumina systems have proven successful for single units, but are being replaced by zirconia and lithium disilicate due to the increased risk of failure in the molar region.24,25 Zirconia can also be used when significant tooth structure is missing, when high risk for flexure and stress is present, for posterior full-crown and fixed partial denture situations (Figure 13 and Figure 14), and when adhesive bonding is problematic, such as with subgingival margins.
In cases where the bond and seal cannot be maintained (ie, high-risk bonding situations, including moisture control problems, high shear and tensile stresses on bonded interfaces, and variable bonding interfaces), high-strength CL-III ceramics or metal ceramics (CL-IV, see below) are appropriate, because they can be placed using conventional cementation techniques. A concern with full-contour zirconia, however, is wear on opposing dentition.26
Whether alumina or zirconia, these materials demonstrate greater strength than CL-I and CL-II materials and can be used to fabricate a core substructure to replace metal. However, they are more opaque due to their greater crystalline content, which detracts from overall esthetics. They are therefore layered with porcelain,27 allowing these materials to offer both superior strength and improved esthetic results.28 CL-III high-strength ceramics require a thickness of 1.2 mm to 1.5 mm, depending on the substrate color.20,25 More translucent versions are now used in the posterior region as full-contour or monolithic all-zirconia restorations. Marketed first in this category was BruxZir® (Glidewell Laboratories, www.bruxzir.com), with many other manufacturers entering the market (Figure 15 and Figure 16).
CL-IV (Metal Ceramics)
CL-IV represents metal ceramics, which are essentially CL-1 materials fused to a highly supportive substrate metal, allowing their use in high-stress clinical situations where conventional crowns and esthetics may be required. They are ideal when minimal-to-no tooth structure remains.
Like CL-III materials, CL-IV metal ceramics demonstrate greater strength but limited esthetic characteristics. CL-IV metal ceramics require a thickness of at least 1.5 mm to create life-like esthetics.28 These metal ceramics demonstrate similar qualities to CL-III zirconia-based restorations, but the metal substructures do not have the same thermal firing sensitivity as zirconia.30
CL-IV metal ceramics can be improved in esthetic qualities with a much higher gold framework material (eg, Captek™, Argen USA Inc., www.captek.com) (Figure 17).
Conclusion
Indications for and composition of today’s dental ceramic materials provide a foundation for determining the appropriate class of ceramics to use for a given case. Other factors that influence material selection include preservation of tooth structure, bond maintenance requirements, esthetics, smile design, and shading.
Both CL-I and CL-II ceramic materials provide high esthetics but limited strength. Although all types of ceramics are weak in tensile and shear stresses compared to compressive stresses, if the stresses can be controlled, weaker materials can be used successfully.7 CL-III and CL-IV ceramic materials offer strength but low esthetic qualities. When functional stresses cannot be controlled and stronger materials (eg, zirconia, alumina, metal) are used, porcelain can be veneered to the substructure for esthetics.
An ideal case would require only one of these ceramic classifications. However, with today’s available material options, delivering restorations that satisfy all requirements is possible.
Disclosure
The authors have no relevant financial affiliations to disclose.
Author Information
Dr. McLaren maintains a private practice limited to prosthodontics and esthetic dentistry in which he does all of his own ceramics. He is the director of the UCLA Center for Esthetic Dentistry, a full time didactic and clinical program for graduate dentists. He is also the founder and director of the UCLA school for Esthetic Dental design. Dr. McLaren has an appointment as an associate professor in the biomaterials and advanced prosthodontic department. He is also an adjunct assistant professor for the University of Oregon Dental School.
Dr. McLaren is a member of numerous associations, including the American College of Prosthodontists, American Academy of Esthetic Dentistry, International Society of Dental Ceramics, International Association of Dental Research, American Association of Dental Research, American Dental Association, and the California Dental Association. He is actively involved in many areas of prosthodontic and materials research, and has published several articles. Dr. McLaren is involved in ongoing clinical research on various restorative systems and has presented numerous lectures, hands-on clinics, and postgraduate courses on ceramics and esthetics across the nation and internationally.
References
1. Leinfelder, KF. Porcelain esthetics for the 21st century. J Am Dent Assoc. 2000;131(1):47S-51S.
2. Ring, ME. Dentistry: An Illustrated History. New York, NY: Harry N. Abrams Inc.,1985.
3. Chu S, Ahmad I. A historical perspective on synthetic ceramic and traditional feldspathic porcelain. Pract Proced Aesthet Dent. 2005;17(9):593-598.
4. Land CH. Porcelain dental art. The Dental Cosmos. 1903;45(6):437-444.
5. McLean JW. The science and art of dental ceramics. A collection of monographs. New Orleans, LA: Louisiana State University School of Dentistry Continuing Education Program, 1976.
6. LeSage BP. Minimally invasive dentistry: paradigm shifts in preparation design. Pract Proced Aesthet Dent. 2009;21(2):97-101.
7. Hondrum SO. A review of the strength properties of dental ceramics. J Prosthet Dent. 1992;67(6):859-865.
8. Calamia JR, Calamia CS. Porcelain laminate veneers: reasons for 25 years of success. Dent Clin North Am. 2007;51(2):399-417.
9. McLaren EA, Cao PT. Ceramics in dentistry–part I: classes of materials. Inside Dentistry. 2009;5(9):433-422.
10. Giordano R. A comparison of all-ceramic systems. J Mass Dent Soc. 2002;50(4):16-20.
11. Calamia JR. Clinical evaluation of etched porcelain veneers. Am J Dent. 1989;2(1):9-15.
12. Kim J, Chu S, Gürel G, Cisneros G.. Restorative space management: treatment planning and clinical considerations for insufficient space. Pract Proced Aesthet Dent. 2005;17(1):19-25.
13. Gürel G. Porcelain laminate veneers: minimal tooth preparation by design. Dent Clin North Am. 2007;51(2):419-431, ix.
14, Kingery WD, Bowen HK, Uhlmann DR. Introduction to Ceramics. 2nd ed. New York, NY: John Wiley and Sons; 1976:1-19.
15. Rosenblum MA, Schulman A. A review of all-ceramic restorations. J Am Dent Assoc. 1997; 128(3):297-307.
16. McLaren EA, Whiteman YY. Ceramics: rationale for material selection. Compend Contin Educ Dent. 2010;31(9):666-668, 670, 672 passim; quiz 680, 700.
17. Mosby’s Dental Dictionary. 2nd ed. St. Louis, MO: Mosby; 2008.
18. Castelnuovo J, Tjan AH, Phillips K, et al. Fracture load and mode of failure of ceramic veneers with different preparations. J Prosthet Dent. 2000;83(2):171-180.
19. Friedman MJ. A 15-year review of porcelain veneer failure—a clinician’s observations. Compend Contin Educ Dent.1998;19(6):625-628, 630, 632 passim; quiz 638.
20. LeSage, B. Revisiting the design of minimal and no-preparation veneers: a step-by-step technique. J Calif Dent Assoc. 2010;38(8):561-569.
21. DiMatteo AM. Prep vs no-prep: the evolution of veneers. Inside Dentistry. 2009;5(6):72-79.
22. Lithium disilicate glass ceramics, United States Patent 6517623. FPO website. www.freepatentsonline.com/6517623.html. Accessed February 4, 2015.
23. Fasbinder DJ, Dennison JB, Heys D, Neiva G. A clinical evaluation of chairside lithium disilicate CAD/CAM crowns: a two-year report. J Am Dent Assoc. 2010;141(suppl 2):10S-14S.
24. Odman P, Andersson B. Procera AllCeram crowns followed for 5 to 10.5 years: a prospective clinical study. Int J Prosthodont. 2001;14(6):504-509.
25. McLaren EA, White SN. Survival of In-Ceram crowns in a private practice: a prospective clinical trial. J Prosthet Dent. 2000;83(2):216-222.
26. Ghuman T, Beck P, Ramp LC, et al. Wear of enamel antagonist to ceramic surfaces. J Dent Res. 2010;89(spec issue B):1394.
27. Pröbster L, Diehl J. Slip-casting alumina ceramics for crown and bridge restorations. Quintessence Int. 1992;23(1):25-31.
28. McLaren EA, Cao PT. Smile analysis and esthetic design: “in the zone”. Inside Dentistry. 2009;5(7):44-48.
29. Augstin-Panadero R, Fons-Font A, Roman-Rodriguez JL, et al. Zirconia versus metal: a preliminary comparative analysis of ceramic veneer behavior. Int J Prosthodont. 2012;25(3):294-300.
30. Chiche G, Pinault A. Esthetics of Anterior Fixed Prosthodontics. Hanover Park, IL: Quintessence Publishing; 1994:13-32.
31. Höland W, Schweiger M, Rheinberger VM, Kappert H. Bioceramics and their application for dental restoration. Adv Appl Ceram. 2009;108(6):373-380.