You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Frequently, pain is the driving force that causes patients to seek dental care. To manage both preoperative discomfort and anticipated postoperative pain, emergency room physicians and dentists alike have historically employed opioids as part of the pharmacologic armamentarium. As a result, many patients have come to expect a prescription for opioids when faced with dental discomfort. This treatment approach, however, has contributed to widespread opiate abuse in the United States. Whether sensationalized or not, anecdotal evidence exists demonstrating that many abusers first encounter opioids through legitimate prescriptions. Published data indicates that patients who received an opioid prescription for dental pain were often given multiple concurrent prescriptions for these drugs within 30 to 180 days.1 Furthermore, dentists prescribe opioids more frequently than other providers for patients aged 10 to 19 years, a population at significant risk for the development of substance abuse disorders.2 Consequently, practitioners must ensure that opioids are prescribed if—and only if—they offer the greatest chance of efficacy despite their inherent risks.
The recent US Surgeon General’s report on addiction prioritizes prevention strategies, and dentists, like other healthcare professionals, face mounting pressure to address the opiate crisis.3 As bodies of evidence exist to support that opioids do not often offer the greatest level of pain control, practitioners should examine their prescribing habits and align them with evidence-based pain management strategies.4 Together with policy-based approaches, these effects may stem the opiate abuse crisis in the United States.
Public Policy Efforts to Reduce Opioid Prescriptions
Large-scale federal and state legislative efforts have been put forth to attempt to tackle the crisis at a policy level. Federal legislation, in the form of S.524, the Comprehensive Addiction and Recovery Act of 2016, was signed into law by President Barack Obama in July 2016.5 It calls for a task force specifically dedicated to establishing best practices for pain management as well as FDA-regulated prescriber education. In addition to these federal efforts, state-specific legislation is underway across the country to help stem the epidemic of opiate abuse. For example, Vermont passed Act 173 in June 2016.6 This act gives specific directives to the Commissioner of Health to more strictly monitor and regulate opioid prescriptions at both provider and dispenser points of contact. The legislation further encourages the Commissioner of Health to adopt specific rules on prescribing opioids following minor medical procedures, including a limit to the maximum number of pills that may be prescribed in a certain time period as well as requisite follow-up examinations prior to dispensing additional medication. Similar legislation is being proposed and passed at the state level nationwide. In addition to state and federal regulations, the US Centers for Disease Control and Prevention has laid out its own recommendations for clinicians to counsel patients and consider alternatives to opioids in pain management when effective and available.3
While these policy-level efforts may successfully effect change in the crisis, practitioners themselves must put forth a concerted effort to reduce unnecessary opioid prescriptions. In light of this, the duty of dental healthcare professionals is to both manage patient discomfort using evidence-based strategies and educate patients about pain management.
Pathophysiology of Odontogenic Pain
To effectively manage pain, one must first understand the generation of dental pain and its perception by the central nervous system. Pulpal and periapical tissues are highly innervated structures that sense and respond to the local environment, including changes caused by dental disease. For example, pulp tissue is replete with both A delta and C fibers. C fibers respond directly to inflammatory mediators, such as those found in irreversible pulpitis, as well as bacteria and their byproducts.7,8 When stimulated, these pulpal nerves, together with neural branches in the periapical tissues, propagate a signal along the maxillary or mandibular branches of the trigeminal nerve through foramina rotunda and ovale, respectively. The neural cell bodies of these first-order sensory neurons are located in the trigeminal ganglion in the middle cranial fossa.9 These fibers then synapse on the subnucleus caudalis in the medullary dorsal horn. Second-order projection neurons cross the midline to their synapse in the thalamus, and then third-order neurons travel to the cerebral cortex. In the cortex, pain is perceived and interpreted.10 Any interruption along the described pathway can be useful in managing discomfort, including both central and peripheral disruption. For example, pain can be altered peripherally by managing infection or inflammation or by altering neural propagation. Central pain management can also be achieved by altering neural responses.
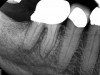
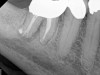
A body of research has emerged in recent years on the management of severe dental pain, exemplified by the diagnosis of an acute apical abscess where drainage cannot be achieved, symptomatic apical periodontitis, or irreversible pulpitis.11 An example of an acute apical abscess is depicted in Figure 1 and Figure 2. These conditions typically represent the most severe discomfort experienced by patients, and consequently, they serve as an excellent model for pain management. Treatment strategies for these issues and other painful conditions should include both definitive treatments for the diagnosed condition as well as adjunctive pharmacologic management.
The primary treatment should, first and foremost, be the definitive treatment of the painful condition, usually by either endodontic therapy or extraction of the affected tooth.12 This is essential, as expeditious definitive treatment will minimize the length of time patients require pain medication. However, adjunctive pharmacologic measures exist, including the use of local anesthetics as well as nonprescription oral analgesics, to control pain when treatment cannot be initiated immediately. Antibiotics should be used judiciously in cases of severe or rapidly progressing infections involving swelling or systemic involvement and will result in reduction in pain as they reduce swelling.13 Antibiotics are not useful in treating irreversible pulpitis or acute pain, and therefore, they should not be relied upon as pain relievers. Their over-prescription could lead to the unintended consequence of increased antibiotic resistance among the general population.
Local Anesthesia
Local anesthesia, a necessary part of definitive treatment, offers targeted pain relief for managing severe discomfort.12 While emergency medical and dental providers historically prescribed opioids when definitive treatment could not be offered immediately, administration of local anesthesia can and should be a viewed as a viable alternative. Local anesthesia can provide patients with several hours of analgesia, without the systemic effects of orally administered analgesics, until that time when definitive dental treatment can be performed.
Local anesthetics interrupt pain processing peripherally by halting the transmission of pain impulses from peripheral neurons to the brain. They block sodium channels in peripheral neurons, preventing depolarization of the affected nerve, thus blocking the pain impulse.14 Infiltration with local anesthetic is useful to manage discomfort in maxillary teeth,15 as well as for mandibular premolars and anterior teeth,16 while inferior alveolar nerve block anesthesia is recommended for mandibular posterior teeth.17 One should note, however, that the success rate for obtaining pulpal anesthesia with an inferior alveolar nerve block alone is relatively low, reported to be between 15% and 57%.17 Therefore, adjunctive anesthesia in addition to the block is often necessary to treat mandibular posterior teeth successfully. According to a randomized control trial, buccal infiltration with articaine proved to be the most successful adjunctive anesthesia technique when combined with an inferior alveolar nerve block.18
Long-acting anesthetics, such as bupivacaine, are useful for increasing anesthesia for several hours beyond the shorter-acting alternatives, but they also demonstrably reduce pain beyond the time of anesthesia.19 Otolaryngologists noted this post-anesthetic effect in children undergoing tonsillectomy and adenoidectomy procedures. When bupivacaine was administered preoperatively, a statistically significant reduction in pain was noted for up to five days postoperatively when compared with patients who were not given the anesthetic.20 This is particularly useful in managing postoperative discomfort following dental treatment. For example, following endodontic surgery where postoperative discomfort is generally of short duration, with maximum discomfort in the immediate postoperative period,21 long-acting anesthetics such as bupivacaine can aid in the management of the most intense periods of pain.12
Alternative Oral Analgesics
Although definitive treatment of dental conditions should be the primary focus of delivering care to patients in pain, and local anesthesia can aid in this regard, management of pain with medications may be advantageous or necessary. Over-the-counter medications useful for managing pain include both non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen. Ibuprofen is the most studied NSAID for dental pain, likely due to its high safety profile, and convenient dosing. Ibuprofen treats inflammation and asserts its effects by blocking the cyclooxygenase 1 and cyclooxygenase 2 enzymes, which prevents the production of prostaglandins involved in pain transmission (Figure 3).12 According to the manufacturer, the maximum recommended dosage of ibuprofen is 3,200 mg/day. Acetaminophen both inhibits prostaglandin synthesis peripherally and acts centrally by interacting with cannabinoid and serotonergic receptors (Figure 4).22 According to the manufacturer, the maximum recommended dosage of acetaminophen is 3,000 mg/day.
Opioids commonly prescribed for dental pain include codeine, hydrocodone, oxycodone, and tramadol. These medications can be prescribed alone, or in combination with acetaminophen. Opioids exert their effects by interacting with Mu and Kappa receptors in the central nervous system and alter pain perception (Figure 5).12 They can create a feeling of euphoria and lessened anxiety, so even though they may not provide complete pain relief, they may allow patients to care less about the pain they are feeling. Opioids are fraught with adverse effects including nausea, vomiting, constipation, psychomotor impairment, and CNS depression.22
While one might assume that a centrally acting drug would offer significantly greater pain relief than their peripherally acting counterparts, the available evidence indicates the converse is true. The Oxford table allows practitioners to systematically compare medication efficacy with respect to moderate to severe pain.4 This table shows that 800 mg of ibuprofen is demonstrably more effective in managing severe dental pain than other available prescription and nonprescription analgesics, including narcotic compounds.4 Furthermore, the combination of ibuprofen and acetaminophen offers greater pain relief than either medication alone and significantly more than combinations of acetaminophen and opioid medication both following endodontic treatment and third-molar extraction.23 A combination of 400 mg of ibuprofen and 500 mg of acetaminophen taken together every six hours offers effective pain relief, while remaining well under the maximum recommended daily dosages of each medication.24 Furthermore, using these lower doses can reduce the side-effect profile, most notably gastrointestinal upset, which is associated with high doses of ibuprofen.25 Using endodontic surgery as an example, nonprescription analgesics, along with the aforementioned long-acting local anesthetics, are otherwise the standard of care.21 Taken together, these findings indicate that, in most instances of endodontic pain, better and more effective alternatives to opioids exist.
Conclusion
A thorough understanding of pain development can permit clinicians to effectively manage their patient’s pain using evidence-based strategies. Skilled delivery of appropriate local anesthesia and detailed instructions on the combined use of ibuprofen and acetaminophen offer effective strategies for the dental practitioner tasked with managing severe dental pain, which are summarized in Figure 6. The research cited here suggests the use of 400 mg of ibuprofen combined with 500 mg of acetaminophen as the first-line strategy for managing dental pain, whether preoperative or postoperative. If this combination is insufficient, increasing the dosage up to 800 mg of ibuprofen is shown to provide the greatest amount of pain relief compared to other orally available prescription and non-prescription analgesics. Local anesthetic can serve as a valuable adjunct or even substitute for oral analgesics, providing targeted and complete pain relief in most circumstances, both before definitive dental care can be provided and afterward.
Given the strength of evidence that opioids are not, in fact, the best means to manage dental pain, as well as new regulations requiring a reduction in their use, clinicians must educate their patients on highly effective opioid alternatives. Reminding patients of the reduced adverse effect profiles, including nausea, vomiting, and constipation that often accompanies opioids, may prove advantageous. Furthermore, a thoughtful discussion of the risks associated with the use of opioid analgesics may discourage their use and, when they are necessary, make patients and caregivers aware of their risks to attenuate potential abuse. Prescribing a pill would often be simpler and involve less patient counseling, but the lasting effects of a thoughtful conversation can result in the prevention of life-changing addiction.
References
1. McCauley JL, Hyer M, Ramakrishnan R, et al. Administrative data from the South Carolina Prescription Drug Monitoring Program. J Am Dent Assoc. 2016:147 (7):537-544.
2. Volkow ND, McLellan TA, Cotto JH, et al. Characteristics of opioid prescriptions in 2009. JAMA. 2011; 305(13):1299-1301.
3. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs and Health. Available at https://addiction.surgeongeneral.gov. Accessed December 3, 2016.
4. Richards D. The Oxford Pain Group League table of analgesic efficacy. Evid Based Dent. 2004;5:22-23.
5. 114th Congress of the United States of America. S.524: Comprehensive Recovery and Addiction Act of 2016. Available at https://www.gpo.gov/fdsys/pkg/BILLS-114s524enr/pdf/BILLS-114s524enr.pdf. Accessed December 13, 2016.
6. Vermont General Assembly. Act No. 173 (S.243). Health; opioids; prescription drugs; Vermont Prescription Monitoring System; acupuncture An act relating to combating opioid abuse in Vermont, 2016. Available at http://legislature.vermont.gov/assets/Documents/2016/Docs/ACTS/ACT173/ACT173%20Act%20Summary.pdf. Accessed December 12, 2016.
7. Olgart L, Kerezoudis NP. Nerve-pulp interactions. Arch Oral Biol. 1994;39 Suppl:47S-54S.
8. Ferraz CCR, Henry MA, Hargreaves KM, Diogenes A. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J Endod. 2011;37:45-48.
9. Fehrenbach MJ, Herring SW. Illustrated anatomy of the head and neck. St. Louis, MO: Elsevier/Saunders; 2012.
10. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
11. Moore A. The evidence base in acute pain. Evid Based Dent. 2000;2:32–33.
12. Hargreaves KM, Keiser K. Building effective strategies for the management of endodontic pain. Endod Topics. 2002;3:93-105.
13. American Association of Endodontists. Endodontics Colleagues for Excellence. Use and Abuse of Antibiotics. Chicago, IL: American Association of Endodontists; 2012.
14. Malamed SF. Handbook of Local Anesthesia. St. Louis, MO: Elsevier/Mosby; 2013.
15. Aggarwal V, Singla M, Miglani S, et al. A prospective, randomized, single-blind comparative evaluation of anesthetic efficacy of posterior superior alveolar nerve blocks, buccal infiltrations, and buccal plus palatal infiltrations in patients with irreversible pulpitis. J Endod. 2011;37:1491-1494.
16. Dressman AS, Nusstein J, Drum M, Reader A. Anesthetic efficacy of a primary articaine infiltration and a repeat articaine infiltration in the incisive/mental nerve region of mandibular premolars: a prospective, randomized, single-blind study. J Endod. 2013;39:313-318.
17. Reader A, Nusstein J, Drum M. Successful local anesthesia for restorative dentistry and endodontics. Chicago: Quintessence Pub. Co., 2011.
18. Kanaa MD, Whitworth JM, Meechan JG. A prospective randomized trial of different supplementary local anesthetic techniques after failure of inferior alveolar nerve block in patients with irreversible pulpitis in mandibular teeth. J Endod. 2012; 38:421-425.
19. Moore PA. Bupivacaine: a long-lasting local anesthetic for dentistry. Oral Surg Oral Med Oral Pathol. 1984; 58:369-374.
20. Jebeles JA, Reilly JS, Gutierrez JF, et al. Tonsillectomy and adenoidectomy pain reduction by local bupivacaine infiltration in children. Int J Pediatr Otorhinolaryngol. 1993;25(1-3):149-154.
21. Chong BS, Pitt Ford TR. Postoperative pain after root-end resection and filling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:762-766.
22. Yagiela JA, Neidle EA, Dowd FJ. Pharmacology and Therapeutics for Dentistry. St. Louis: Mosby, 1998.
23. Moore PA, Hersch EV. Combining ibuprofen and acetaminophen for acute pain management after third molar extractions: Translating clinical research to dental practice. J Am Dent Assoc. 2013;144(8):898-908.
24. Menhinick KA, Gutmann J, Regan JD, et al. The efficacy of pain control following nonsurgical root canal treatment using ibuprofen or a combination of ibuprofen and acetaminophen in a randomized, double-blind, placebo-controlled study. Int Endodo J. 2004;37:531-41.
25. Hargreaves KM, Troullos ES, Dionne RA. Pharmacologic rationale for the treatment of acute pain. Dent Clin N Am. 1987;31:675-694.
About the Authors
Brooke Blicher, DMD
Upper Valley Endodontics, PC
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Clinical Instructor
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Rebekah Lucier Pryles, DMD
Upper Valley Endodontics, PC
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts