You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
MSEO is a poorly understood, difficult to treat pathologic entity that results when apical periodontitis spreads from the maxillary posterior dentition to the sinus, causing additional sinus pathosis.9 Its development is facilitated by the intimate anatomic relationships between the root apices of the maxillary posterior teeth and the sinus floor.13-16 The root apices may sit in contact with the sinus floor, lie laterally alongside it, or project into the sinus floor itself.14 The teeth most closely related to the maxillary sinuses are the maxillary second molar, first molar, third molar, and second premolar, respectively.13,15,16 The apices of the maxillary second molar protrude into the sinus in as many as 45% of patients.14,15 The proximity of these structures provides a pathway for odontogenic infections and local inflammatory mediators to spread to the sinus.
Etiology and Pathogenesis of Odontogenic Sinus Disease
Although the spread of infection from one area to the next is readily understandable, the nature of sinus pathosis can take many forms. There are many variations within sinus presentations (Figure 1). Rhinosinusitis refers to inflammation of the nose and paranasal sinuses. This term is currently preferred over sinusitis by ear, nose, and throat (ENT) specialists because rhinitis and sinusitis are concurrent in most individuals.17 Chronic rhinosinusitis (CRS) is defined as ongoing rhinosinusitis that lasts for more than 12 weeks.18 CRS develops when the Schneiderian membrane lining the sinus is compromised, for example, as a result of periodontal surgery, sinus floor elevation, tooth extraction, trauma, entry of a foreign body, malignancy, or exposure to inflammatory mediators from an odontogenic or periodontal infection.4,7 CRS is the diagnosis most often related to odontogenically derived sinus infections due to chronic exposure to the infection source. Rhinosinusitis with an odontogenic source is referred to variably in the literature as odontogenic maxillary sinusitis (OMS), odontogenic sinusitis, odontogenic rhinosinusitis, and maxillary sinusitis of dental origin.9 MSEO, a term coined by the AAE in 2018, refers specifically to rhinosinusitis that develops secondary to endodontic pathosis.9
Because MSEO results from the spread of pulpal infection into periapical tissues and ultimately into the sinus,9 the microbial communities associated with MSEO are similar to those associated with pulp necrosis and apical periodontitis. These infections are polymicrobial, including both aerobic and anaerobic species, with apredominance of anaerobes.5 Fungal species, such as Aspergillus, have also been implicated in MSEO.8 Bacterial virulence factors, lysosomal enzymes, and toxins promote microbial invasion and tissue breakdown in the periapical bone adjacent to the cortical floor of the sinus.
Local inflammation and infection in the alveolar bone and periodontium has been correlated with a thickening of the soft-tissue mucosal barrier that can be visualized on CBCT scans. This thickening is referred to as "mucositis."6,13,19 A recent study found that pathologic mucositis was more than 9.75× more likely to be observed among teeth with periapical lesions than without.6 Mucosal thickening of greater than 2 mm is considered pathologic6,13 due to its potential to diminish mucociliary clearance and facilitate the growth of pathogenic bacteria.19 The resulting inflammation can cause closure of the ostiomeatal complex, ultimately leading to further mucosal swelling, the accumulation of purulence, and the development of an anaerobic environment. Together, these processes cumulate in the development of rhinosinusitis.8 In cases of CRS, mucositis averages 7.4 mm in thickness20 and is correlated with MSEO, periodontal disease, root canal treatment, edentulism, and proximity of the root apex to the sinus.6,13
Epidemiology of Sinus Disease
CRS is a common condition that affects approximately 12% of the US population annually,18 most often those aged 30 to 50.4 If left untreated, CRS can progress to pansinusitis, which can spread into orbital and cranial structures and potentially lead to cavernous sinus thrombosis, osteomyelitis, or meningitis.5,8 The incidence of OMS is unknown. Until recently, research indicated that 10% to 12% of sinusitis cases were of odontogenic origin. However, more recent data suggest that 24% to 51% of CRS cases develop secondary to dental disease.3,20-22 OMS is most often caused by dentoalveolar surgical interventions, followed by MSEO. Periodontal pathosis and implant-associated OMS are less common.8,23
Diagnosis of MSEO
Because dental pathosis may account for as many as 75% to 80% of unilateral CRS cases,1,11,23 patients with persistent CRS of unknown or suspected dental etiology should be evaluated for dental disease.2,17,24 That being said, cases of unilateral CRS must be carefully diagnosed because several potentially dangerous entities also cause this pathosis. The differential diagnosis for unilateral CRS includes OMS, CRS with nasal polyps, aspergilloma, allergic fungal sinusitis, benign tumors, carcinoma, malignancy, and antrochoanal polyps, including inverted papillomas.18,25 Clinical signs and symptoms often associated with these more severe pathologies include osseous destruction, extra-sinus expansion or local invasion, facial swelling, cranial nerve palsies, proptosis, and severe headache.17,18 When there is complete unilateral opacification with expansion of the sinus noted on imaging, a diagnosis of maxillary sinus mucocele is given, which requires urgent treatment.26
Despite the reportedly high incidence of OMS, odontogenic findings are rarely discussed on sinus imaging ordered by ENT specialists. A study conducted in 2011 revealed that dental pathosis was reported in only 30% of the initial radiology reports of patients with OMS. Medical guidelines for sinus conditions rarely mention dental causes of rhinosinusitis, and even fewer guidelines recommend a dental evaluation.21 Data published in 2012 suggest that many radiologists and ENT specialists may be unaware of the relationship between apical periodontitis and sinusitis.2
Unfortunately for diagnosing clinicians, the symptoms of OMS and MSEO do not differ from non-odontogenic CRS. They include sinonasal symptoms such as nasal obstruction, rhinorrhea, facial pain, headache, foul odor, foul taste, and postnasal drip.11,20 Dental symptoms cannot be used to reliably predict an odontogenic cause of rhinosinusitis.27,28 In the absence of dental disease, non-odontogenic sinusitis can still present with dental symptoms, including pain from thermal stimuli and mastication.2,12,28 Conversely, dental pain may not be present in patients with MSEO due to the release of pressure into the sinus.9,12 According to the results of one study, dental pain was reported in only 29% of patients with proven OMS.2
The diagnostic workup for suspected MSEO requires both clinical and radiographic exams. The clinical exam for suspected MSEO should involve pulp sensitivity testing; periapical testing, including percussion and palpation; and a periodontal exam, including periodontal probing and mobility tests. A pulpal diagnosis of necrosis or previously endodontically treated and a periapical diagnosis of symptomatic or asymptomatic apical periodontitis or chronic or acute apical abscess will likely be associated with MSEO.9
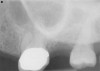
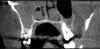
Because of its high resolution and ability to discern between bone and soft tissue, CBCT is the gold standard radiographic exam for the diagnosis of MSEO.2,12,18,20 CBCT imaging provides several advantages over traditional 2-dimensional dental radiographs. On periapical radiographs, anatomic noise imparted by the overlapping structures of the maxillary sinus and zygomatic process can conceal the inflammatory changes associated with apical periodontitis.9,29 Furthermore, 2-D radiographs do not adequately describe the anatomical relationship between the teeth and the sinus floor (Figure 2).3,9
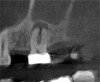
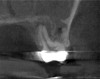
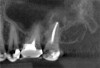
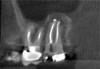
For patients suffering from MSEO, radiographs will reveal a periapical radiolucency with adjacent opacification in the maxillary sinus.12 Additional radiographic findings associated with MSEO include periapical mucositis and periapical osteoperiostitis (PAO). Periapical mucositis refers to mucosal thickening or dome-shaped soft-tissue expansion on the floor of the sinus directly adjacent to the infected root apex.9 PAO refers to the reactive osteogenesis caused by a local periosteal reaction that expands the sinus periosteum and displaces it upward into the sinus. It appears as a radiopaque "halo" surrounding the root apex (Figure 2 through Figure 4). PAO may be symptomatic and/or accompanied by adjacent mucosal edema and elevated sinus fluid levels.9 If left untreated, PAO can progress and result in a direct communication between the root apex and maxillary sinus.6,13 Unless bilateral odontogenic pathosis is found, the sinus opacifications will usually be unilateral and centered around the odontogenic source. Complete unilateral sinus opacifications may be missed on the CBCT images typically used in endodontics due to their limited or focused field of view (Figure 5). In cases involving complete unilateral sinus opacification, referral to an ENT or oral surgeon is essential to rule out invasive fungal infection or malignancy.2,17,24
Treatment of MSEO
Multidisciplinary treatment of OMS is necessary to manage both the dental source and the related sinus disease. Best-practice guidelines for treatment sequencing are currently under development.11,12 Very often, treatment strategies center primarily on physical treatment of the dental pathosis and include concomitant medical or surgical management.
When an odontogenic source of infection is diagnosed, physical treatment of the odontogenic infection with appropriate clinical and radiographic follow-up is indicated.2,8,9,11 Dental treatment alone can significantly improve sinus symptoms and may resolve the disease.9
Dental treatment for MSEO should be selected based on its ability to appropriately manage the odontogenic source of infection, namely the diseased pulp or reinfected pulp space. Treatment modalities can include nonsurgical root canal therapy (NSRCT), nonsurgical re-treatment, periradicular surgery, intentional replantation, or extraction.9 Some data suggest that extraction should be avoided in MSEO treatment due to the high risk of oroantral fistula development. In a recent study of third molar extractions, a higher frequency of sinus communications was noted in patients who were suffering from sinusitis at the time of extraction.30 Sinusitis also complicates healing after a sinus communication, and a high risk of recurrence has been noted.12
Medical management of CRS is often necessary in conjunction with dental treatment. The current guidelines for medical management of CRS recommend nasal saline irrigation and topical intranasal corticosteroids as a first-line therapy.17,19,31 Currently, there is no high-level evidence to support the use of systemic antibiotics in the management of CRS.17-19,24 For MSEO, the AAE and other researchers note that antibiotics are "utterly ineffective as a definitive solution,"9 but are sometimes used in combination with other appropriate treatments. Antibiotics can temporarily improve sinus symptoms and may be indicated in cases involving rapidly spreading infections.9,17
For patients with minor sinus disease, treatment of the dental etiology with concomitant medical management may suffice to resolve the pathosis. However, patients with significant sinus disease are unlikely to achieve full resolution without both dental treatment and sinus surgery in coordination.12 In these cases, failure to address the odontogenic source of infection increases the risk that the surgical sinus intervention will fail.19 If sinusitis symptoms persist, surgical treatment of the resulting sinus condition may be necessary to restore appropriate sinus drainage.2,8,9,11
Functional endoscopic sinus surgery (FESS) is well-established for the treatment of CRS refractory to dental or medical treatment. FESS is used to restore ventilation and drainage through the ostiomeatal complex,26 and can be performed under local or general anesthesia. The procedure often includes maxillary antrostomy, which is an opening and enlargement of the natural ostium. Anterior ethmoidectomy (ie, resection and debridement of the ethmoid bulla and air cells), posterior ethmoidectomy, or sphenoidotomy (ie, enlargement of the sphenoid ostium) are also performed as needed. Patients are discharged with nasal spray and antibiotics, as indicated.32
Sinusitis caused by larger foreign bodies is often treated by an intraoral approach known as the Caldwell-Luc procedure or radical antrostomy. FESS is currently preferred over Caldwell-Luc because it is less invasive and has less risk of complications.33 Such surgeries are typically followed by adjunctive pharmacologic treatment, and it may take several weeks for complete symptom resolution.26,33 The dental, medical, and surgical care required to appropriately treat MSEO is highlighted in the following case.
Case Report
A 58-year-old female patient presented to the office with a 3-month history of acute sinusitis, during which six courses of antibiotics, including penicillin, cephalosporin, and quinolone drugs, had failed to resolve her symptoms. She had been referred for endodontic evaluation by her ENT specialist after imaging revealed endodontic pathosis of tooth No. 2. On initial presentation, tooth No. 2 was nonvital (ie, nonresponsive to cold or electric pulp testing), tested positive to percussion and palpation, and was without swelling, sinus tracts, or periodontal defects. The preoperative CBCT scan revealed apical pathosis on the buccal and palatal roots of tooth No. 2, complete opacification of the right maxillary and ethmoid sinuses, and disruption of the lateral/posterior antral wall posterior to the zygomaticomaxillary buttress (Figure 6). Root canal therapy was initiated. Upon access, black staining of the pulp tissue was observed, indicating necrosis. Pulpal debridement was performed, and calcium hydroxide was utilized as an intracanal medication. Several weeks after pulpal debridement, the patient's sinus symptoms improved, but incompletely. Tooth No. 2 was obturated, and the patient was referred to an ENT specialist for further care.
The ENT specialist performed an endoscopic procedure, noting white mucopurulent drainage from the middle meatus, after which the patient was prescribed a topical decongestant to be taken for 2 weeks. At a follow-up appointment 2 months later, the patient reported that she continued to experience persistent sinus symptoms with slow improvement. At that time, she agreed to proceed with FESS, and a right-side nasal endoscopy with debridement was performed. At her next 3-week follow-up appointment, she noted that she continued to experience sinus pain and pressure; therefore, she was treated with an additional course of antibiotics.
Three months after NSRCT and one month after FESS, she returned to her endodontist for a follow-up appointment. She reported experiencing only minor sinus pressure, which she believed to be attributed to seasonal allergies. Upon examination, no swelling, mobility, or sinus tracts were noted, and tooth No. 2 was not tender to percussion or palpation. CBCT imaging revealed both clearing of the sinus and initial healing of the apical pathosis (Figure 7 and Figure 8). Continued follow-up appointments were recommended.
This case demonstrates a typical presentation of MSEO, in which an apical radiolucency obliterated the cortical bone of the sinus, causing a direct communication between the two structures. NSRCT was used to treat the dental etiology and following resolution of the apical pathosis, resulted in the restoration of the cortical boundary between the root apex and maxillary sinus. The severity of the pathosis necessitated significant sinus treatment to achieve resolution, including dental, medical, and surgical interventions.
Conclusion
MSEO provides practitioners with a unique diagnostic challenge. The anatomic proximity of the maxillary dentition to the maxillary sinus permits the development of communicating pathologies. Because the symptoms of nonodontogenic and odontogenic CRS are indistinguishable, patients presenting with CRS, particularly in cases of unilateral sinus disease, should be evaluated for dental pathosis of the maxillary dentition. Advances in imaging, such as those provided by CBCT, facilitate accurate diagnosis of these pathologic entities. Once a diagnosis of MSEO is established, treatment should be coordinated with ENT specialists to address the dental source of the disease as well as the resulting sinus pathosis.
About the Authors
Jessica Langella
DMD Candidate, Class of 2019
Harvard School of Dental Medicine
Boston, Massachusetts
Brooke Blicher, DMD
Upper Valley Endodontics
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Clinical Instructor
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Rebekah Lucier Pryles, DMD
Upper Valley Endodontics
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
References
1. Nunes CA, Guedes OA, Alencar AH, et al. Evaluation of periapical lesions and their association with maxillary sinus abnormalities on cone-beam computed tomographic images. J Endod. 2016;42(1):42-46.
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head and Neck Surg. 2012;20(1):24-28.
3. Bomeli SR, Branstetter BF 4th, Ferguson BJ. Frequency of a dental source for acute maxillary sinusitis. Laryngoscope. 2009;119(3):580-584.
4. Lechien JR, Filleul O, Costa de Araujo P, et al. Chronic maxillary rhinosinusitis of dental origin: a systematic review of 674 patient cases. Int J Otolaryngol. 2014;2014:465173.
5. Mehra P, Jeong D. Maxillary sinusitis of odontogenic origin. Curr Allergy Asthma Rep. 2009;9(3):238-243.
6. Shanbhag S, Karnik P, Shirke P, et al. Association between periapical lesions and maxillary sinus mucosal thickening: a retrospective cone-beam computed tomographic study. J Endod. 2013;39(7):853-857.
7. Taschieri S, Torretta S, Corbella S, et al. Patho-physiology of sinusitis of odontogenic origin. J Investig Clin Dent. 2017;8(2). doi: 10.1111/jicd.12202.
8. Zirk M, Dreiseidler T, Pohl M, et al. Odontogenic sinusitis maxillaris: A retrospective study of 121 cases with surgical intervention. J Craniomaxillofac Surg. 2017;45(4):520-525.
9. Tataryn R, Lewis M, Horalek A, et al. Maxillary sinusitis of endodontic origin: AAE position statement. AAE Board of Directors. 2018:1-11.
10. Longhini AB, Branstetter BF, Ferguson BJ. Otolaryngologists' perceptions of odontogenic maxillary sinusitis. Laryngoscope.2012;122(9):1910-1914.
11. Matsumoto Y, Ikeda T, Yokoi H, et al. Association between odontogenic infections and unilateral sinus opacification. Auris Nasus Larynx. 2015;42(4):288-293.
12. Workman AD, Granquist EJ, Adappa ND. Odontogenic sinusitis: developments in diagnosis, microbiology, and treatment. Curr Opin Otolaryngol Head Neck Surg. 2018;26(1):27-33.
13. Aksoy U, Orhan K. Association between odontogenic conditions and maxillary sinus mucosal thickening: a retrospective CBCT study. Clin Oral Investig. 2018. doi: 10.1007/s00784-018-2418-x.
14. Jung YH, Cho BH. Assessment of the relationship between the maxillary molars and adjacent structures using cone beam computed tomography. Imaging Sci Dent. 2012;42(4):219-224.
15. Kang SH, Kim BS, Kim Y. Proximity of posterior teeth to the maxillary sinus and buccal bone thickness: a biometric assessment using cone-beam computed tomography. J Endod. 2015;41(11):1839-1846.
16. Tian XM, Qian L, Xin XZ, et al. An analysis of the proximity of maxillary posterior teeth to the maxillary sinus using cone-beam computed tomography. J Endod. 2016;42(3):371-377.
17. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1-12.
18. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
19. Kohanski MA, Toskala E, Kennedy DW. Evolution in the surgical management of chronic rhinosinusitis: current indications and pitfalls. J Allergy Clin Immunol. 2018;141(5):1561-1569.
20. Maillet M, Bowles WR, McClanahan SL, et al. Cone-beam computed tomography evaluation of maxillary sinusitis. J Endod. 2011;37(6):753-757.
21. Longhini AB, Ferguson BJ. Clinical aspects of odontogenic maxillary sinusitis: a case series. Int Forum Allergy Rhinol. 2011;1(5):409-415.
22. Vestin Fredriksson M, Ohman A, Flygare L, et al. When maxillary sinusitis does not heal: findings on CBCT scans of the sinuses with a particular focus on the occurrence of odontogenic causes of maxillary sinusitis. Laryngoscope Investig Otolaryngol. 2017;2(6):442-446.
23. Troeltzsch M, Pache C, Troeltzsch M, et al. Etiology and clinical characteristics of symptomatic unilateral maxillary sinusitis: A review of 174 cases. J Craniomaxillofac Surg. 2015;43(8):1522-1529.
24. Adelson RT, Adappa ND. What is the proper role of oral antibiotics in the treatment of patients with chronic sinusitis? Curr Opin Otolaryngol Head Neck Surg. 2013;21(1):61-68.
25. Lee JY. Unilateral paranasal sinus diseases: analysis of the clinical characteristics, diagnosis, pathology, and computed tomography findings. Acta Otolaryngol. 2008;128(6):621-626.
26. Dhillon RS, East CA. Ear, Nose and Throat and Head and Neck Surgery: An Illustrated Colour Text. 4th ed: Elsevier; 2012.
27. Chen YH, Tseng CC, Chao WY, et al. Toothache with a multifactorial etiology: a case report. Dental traumatology. 1997;13(5):245-247.
28. Yatani H, Komiyama O, Matsuka Y, et al. Systematic review and recommendations for nonodontogenic toothache. J Oral Rehabil. 2014;41(11):843-852.
29. Low KM, Dula K, Burgin W, et al. Comparison of periapical radiography and limited cone-beam tomography in posterior maxillary teeth referred for apical surgery. J Endod. 2008;34(5):557-562.
30. Hasegawa T, Tachibana A, Takeda D, et al. Risk factors associated with oroantral perforation during surgical removal of maxillary third molar teeth. Oral Maxillofacl Surg. 2016;20(4):369-375.
31. Beswick DM, Mace JC, Chowdhury NI, et al. Comparison of surgical outcomes between patients with unilateral and bilateral chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(12):1162-1169.
32. Patel A. Functional Endoscopic Sinus Surgery. Medscape Website.https://emedicine.medscape.com/article/863420-overview. Updated March 2, 2016. Accessed September 26, 2018.
33. Tanasiewicz M, Bubilek-Bogacz A, Twardawa H, Skucha-Nowak M, et al. Foreign body of endodontic origin in the maxillary sinus. Journal of Dental Sciences. 2017;12(3):296-300.