You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
In providing conventional endodontic treatment to patients, a pulpectomy is performed to treat the inflamed or infected pulpal tissue. This treatment involves the complete extirpation of the pulp tissue, both necrotic and vital, via a chemomechanical (ie, sodium hypochlorite, ethylenediaminetetraacetic acid [EDTA], endodontic files) process. This technique also involves obtaining the correct endodontic file working length of a canal and then working the canal up to the largest file size (ie, master apical file [MAF]) that is able to be placed to the specified working length. After the canal is properly prepared (ie, cleansed, shaped), obturation of the canal is completed by placing biocompatible foreign materials (eg, gutta-percha, sealer) to the working length.
Historically, canal obturation has been performed with gutta-percha and sealer in order to obtain a proper seal.1 Gutta-percha has been used in endodontics since the 1860s.2 Because gutta-percha does not adhere to dentin, it must be used in combination with a sealer cement to fill any voids and gaps between the root dentin and filling material. Therefore, excellent sealing ability and marginal adaptation are the two most important criteria for an ideal endodontic obturation material.3 Bioactive endodontics eliminates all of these clinical obturation steps by using bioactive materials and the patient's own blood in order to regenerate tissue in the root canal system.
By definition, bioactive means having a biological effect. The bioactive tissues and materials used in regenerative endodontics, such as clotted blood, bioceramics, and ETDA, have all demonstrated a biological effect after usage in treatment.4 This technique represents a paradigm shift from the current conventional endodontic procedural techniques. It does not require the clinician to maintain the meticulous working length measurements (ie, 0.5-mm increments) from MAF to master apical cone placement. It also eliminates the need for warm vertical and cold lateral compaction techniques along with carrier-based root-filling materials for canal obturation. In addition, when a clinician performs regenerative endodontics, the possibility of canal obturation overfills and underfills is also eliminated.
Regenerative Endodontics
Regenerative endodontics is defined as the performance of biologically-based procedures designed to physiologically replace damaged tooth structures, including dentin and root structures as well as the pulp-dentin complex.5 It should be noted that throughout the endodontic literature, the terms regeneration, revascularization, and revitalization are used synonymously and interchangeably.4 Clinically, regenerative endodontics was first developed for the treatment of necrotic immature permanent teeth in order to obtain root end closure and encourage continued development of the root and thickening of the canal walls.6,7 In addition to treating immature permanent teeth with necrotic pulps, reports in the literature have also demonstrated the use of regenerative endodontic procedures on mature permanent teeth with necrotic pulps, teeth with persistent apical periodontitis after conventional endodontic treatment, traumatized teeth with external inflammatory resorption, teeth with horizontal fractures, and avulsed teeth.8-10 Regenerative endodontics has also been performed on vital mature permanent teeth (ie, those with a pretreatment pulpal diagnosis of symptomatic irreversible pulpitis).
Regenerative Endodontic Treatment for Necrotic Mature Permanent Teeth
After obtaining a proper pretreatment pulpal and periradicular diagnosis that includes current radiographs (eg, periapical, bitewings, cone-beam computed tomography [CBCT]), the tooth to be treated is anesthetized and a rubber dam is placed. Canal access is achieved, and hand files (eg, No. 10/02, No. 15/02) are used in combination with an apex locator instrument to determine the canal working length. Regarding canal preparation, although there is not a specific endodontic canal preparation technique required for the performance of regenerative endodontics, there are some canal preparation guidelines.11-13 First, the coronal two-thirds of the canal does not need to be overly enlarged. The reason for this is that the dentist does not have to be concerned with this portion of the canal being large enough to allow obturation instruments access to the apical third of the canal. This conservative enlargement of the coronal two-thirds of the canal will also do less to weaken the tooth and thus prevent root fractures.14 Second, the final canal size of the apical foramen needs to accommodate, at a minimum, an MAF of 0.32 mm in order to allow the cells of the periapical tissue to migrate into the canal space.15 And last, during the filing stage of canal preparation, the canal should be irrigated with a 1.5% sodium hypochlorite solution (a lower concentration is used to reduce the killing of stem cells),16 and an EDTA gel should be placed on each file prior to its introduction into the canal.17
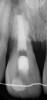
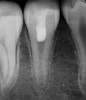
After the canal preparation is completed, it is irrigated with 17% EDTA and dried. Calcium hydroxide is then placed to serve as an antimicrobial medication between treatments (Figure 1).18,19 The canal access is temporized with a sterile cotton pellet or sponge and a temporary restorative material or glass ionomer. The patient is then asked to return in 1 to 4 weeks for the final treatment appointment.15
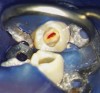
At the final regenerative endodontic treatment appointment, a 3% mepivacaine solution without a vasoconstrictor is used to obtain local anesthesia (the lack of a vasoconstrictor will allow for periapical bleeding). A rubber dam is placed, and after the tooth is accessed, 17% EDTA is used to irrigate the canal in conjunction with files to remove the calcium hydroxide and prepare the dentin. Next, the canal is dried, and bleeding is induced with a hand file 1 to 2 mm beyond the apical foramen. The blood should be stopped at a level in the canal (ie, cementoenamel junction) that will allow for the placement of 3 to 4 mm of restorative material (Figure 2). The induction of periapical bleeding into the canal space is necessary in regenerative endodontic procedures. The induced bleeding supplies scaffolding, stem cells, and blood-derived bioactive growth factors. These growth factors supplement the growth factors embedded in the dentinal matrix that are released when the 17% EDTA is irrigated into the canal.20-22 It has been shown that the stem cells from the apical papilla and the growth factors released from the dentin demonstrate an increased survival rate with the use of EDTA and calcium hydroxide.22 In contrast, the use of high concentrations of sodium hypochlorite and antibiotic paste have been shown to result in decreased stem cell and growth factor survival.23 The triad of stem cells, scaffolding, and growth factors is what enables the tissue regeneration to occur.24
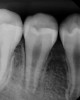
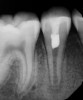
Following the induction of bleeding, a resorbable collagen matrix is placed over the blood clot. Next, a bioceramic material is placed directly over the collagen matrix, a glass ionomer is placed over the bioceramic material, and a final composite or amalgam restoration is placed over the glass ionomer. Once the restoration is complete, a final radiograph is taken (Figure 3 and Figure 4), and the patient is put on 6-month recall for up to 3 years, as dictated by the healing process (Figure 5).25
When performing regenerative endodontic treatment on necrotic mature permanent teeth, the use of two treatment appointments is recommended.26 This is because it is paramount that a necrotic pulp infection is properly removed in order for the regenerative endodontic procedure to be effective. In addition, thickening of the canal walls and continued root development, which is often observed in regenerative endodontic treatment for necrotic immature permanent teeth, does not occur in mature permanent teeth; however, apical closure can occur.27 It is also important to note that, unlike in regenerative endodontic treatment on necrotic immature permanent teeth where it is recommended to perform minimal canal preparation because of the thin dentinal walls, regenerative endodontic treatment for necrotic mature permanent teeth necessitates complete mechanical debridement to remove the necrotic tissue and eliminate root canal infection.15
Regenerative Endodontic Technique for Vital Mature Permanent Teeth
Performing regenerative endodontic procedures on vital mature permanent teeth involves the exact same treatment steps that are used for necrotic mature permanent teeth, except that in vital teeth, the bacterial infection component that is observed in necrotic pulp cases is not the same (ie, no bacteria to minimal pulpal bacteria is present).28,29 Therefore, these teeth do not require the placement of calcium hydroxide and can be treated in a single visit. In addition, in a study by Wang and colleagues,30 the existence of functional stem cells in clinically compromised dental pulp with irreversible pulpitis was demonstrated. It is important to note that this procedure is just beginning to be used clinically; therefore, robust peer-reviewed studies are not yet available in the literature.
Bioactive Effects
The bioactive effects from regenerative endodontics have demonstrated the ability to repair the damaged pulp tissue; however, they do not result in actual regeneration.4 The tissues generated in a canal after a regenerative endodontic procedure has been performed are cementum-like, bone-like, periodontal ligament-like tissues, complete with blood vessels and nerves. Although these tissues are not true pulpal tissue, they are the host's own vital tissues, so unlike foreign obturation materials (eg, gutta-percha, sealer), they are inherited with immune defense mechanisms to protect the canal system from infection.15,26,28
According to the American Association of Endodontists,26 there are three measurable goals to gauge the success of a regenerative endodontic procedure. The primary goal is to eliminate the patient's symptoms and demonstrate evidence of bony healing (if applicable). The secondary goal is to achieve an increase in root wall thickness and/or root length in cases involving permanent teeth with immature apices. The tertiary goal is to attain a positive response to vitality testing. This is possible because although the pulp replacement tissues are not actual dental pulp, they may still have sensory nerve fibers that will respond to sensibility testing (eg, cold/electric pulp tester).15
Conclusion
Bioactive treatment is the future direction of endodontics. This treatment involves the performance of various regenerative endodontic techniques, which provide a more biologic approach to endodontic treatment than the current, conventional clinical methodology. Further clinical studies are needed to observe the long-term prognosis when bioactive endodontic procedures are performed.
Acknowledgment
The authors would like to thank Julia Nguyen, DDS, and Daniel Oh, DDS, at the University of Illinois at Chicago, College of Dentistry, for the clinical treatment and documentation of the regenerative endodontic procedures.
About the Author
James Bahcall, DMD, MS
Clinical Professor
Department of Endodontics
University of Illinois at Chicago
College of Dentistry
Chicago, Illinois
Qian Xie, DDS, PhD
Assistant Professor
Department of Endodontics
University of Illinois at Chicago
College of Dentistry
Chicago, Illinois
Mark Baker, DDSClinical Associate Professor
Department of Endodontics
University of Illinois at Chicago
College of Dentistry
Chicago, Illinois
Steve Weeks, DDS
Clinical Assistant Professor
Department of Endodontics
University of Illinois at Chicago
College of Dentistry
Chicago, Illinois
Vikash Huliyar, DDS
Second-Year Endodontic Resident
University of Illinois at Chicago
College of Dentistry
Chicago, Illinois
References
1. Swartz DB, Skidmore AE, Griffin JA Jr. Twenty years of endodontic success and failure. J Endod. 1983;9(5):
198-202.
2. Friedman CM, Sandrik JL, Heuer MA, et al. Composition and mechanical properties of gutta-percha endodontic points. J Dent Res. 1975;54(5):921-925.
3. Al-Kahtani AM. Carrier-based root canal filling materials: a literature review. J Contemp Dent Pract. 2013;14
(4):777-783.
4. Kim SG, Malek M, Sigurdsson A, et al. Regenerative endodontics: a comprehensive review. Int Endod J. 2018;51(12):1367-1388.
5. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(4):377-90.
6. Ostby BN. The role of the blood clot in endodontic therapy. An experimental histological study. Acta Odontol Scand. 1961;19:324-353.
7. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196-200.
8. Saoud TM, Martin G, Chen YH, et al. Treatment of mature permanent teeth with necrotic pulps and apical periodontitis using regenerative endodontic procedures: a case series. J Endod. 2016;42(1):57-64.
9. Saoud TM, Huang GT, Gibbs JL, et al. Management of teeth with persistent apical periodontitis after root canal treatment using regenerative endodontic therapy. J Endod. 2015;41(10):1743-1748.
10. Santiago CN, Pinto SS, Sassone LM, et al. Revascular-ization technique for the treatment of external inflammatory root resorption: a report of 3 cases. J Endod. 2015;41(9):1560-1564.
11. Siddique R, Nivedhitha MS. Effectiveness of rotary and reciprocating systems on microbial reduction: A systematic review. J Conserv Dent. 2019;22:114-122.
12. Weeks S, Bahcall J. Continuous or Reciprocating Endodontic Rotary Files Evidence-Based Clinical Considerations. Dentistry Today. 2017;36(10):125-127.
13. Molina B, Glickman G, Vandrangi P, et al. Evaluation of Root Canal Debridement of Human Molars Using GentleWave System. J Endod. 2015;41(10):1701-1705.
14. Khoshbin E, Donyavi Z, Abbasi Atibeh E, et al. The effect of canal preparation with four different rotary systems on the formation of dentinal cracks: an in vitro evaluation. Iran Endod J. 2018;13(2):163-168.
15. Saoud TMA, Ricucci D, Lin LM, et al. Regeneration and repair in endodontics-a special issue of the regenerative endodontics-a new era in clinical endodontics. Dent J (Basel). 2016;4(1). pii: E3.
16. Gallar KM, Buchalla W, Hiller KA, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41(3):363-368.
17. Bahcall J, Johnson B. Clinical guide to treating endodontic emergencies. Inside Dentistry. 2016;12(4):46-51.
18. Martin DE, De Almeida JF, Henry MA, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40(1):51-55.
19. Mohammadi Z, Drummer PM. Properties and application of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44(8):697-730.
20. Casagrande L, Demarco FF, Zhang Z, et al. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89(6):603-608.
21. Cao Y, Song M, Kim E, et al. Pulp-dentin regeneration: current state and future prospects. J Dent Res. 2015;
94(11):1544-1551.
22. Galler KM, Buchalla W, Hiller KA, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41(3):363-368.
23. Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014;40(4):521-525.
24. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920-926.
25. Kahler B, Mistry S, Moule A, et al. Revascularization outcomes: a prospective analysis of 16 consecutive cases. J Endod. 2014;40(3):333-338.
26. American Association of Endodontists. Clinical considerations for a regenerative procedure. American Association of Endodontists web site. https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/specialty/wp-content/uploads/sites/2/2018/06/ConsiderationsForRegEndo_AsOfApril2018.pdf. Revised April 1, 2018. Accessed April 22, 2019.
27. Saoud TM, Sigurdsson A, Rosenburg PA, et al. Treat-ment of a large cystlike inflammatory periapical lesion associated with mature necrotic teeth using regenerative endodontic therapy. J Endod. 2014;40(12):2081-2086.
28. Nagaoka S, Miyazaki Y, Liu HJ, et al. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J Endod. 1995;21(2):70-73.
29. Ricucci D, Loghin S, Siqueira JF Jr. Correlation between clinical and histological pulp diagnosis. J Endod. 2014;40(12):1932-1939.
30. Wang Z, Pan J, Wright JT, et al. Putative stem cells in human dental pulp with irreversible pulpitis: an exploratory study. J Endod. 2010;36(5):820-825.