You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Bone Graft Materials for Lateral Sinus Augmentation
Lateral sinus augmentation is a surgical procedure that is used to increase vertical bone height and bone volume in the posterior region of the maxilla in order to facilitate the placement of implants for the rehabilitation of edentulous areas. Sinus bone grafting was initially described by Boyne and James in 1980.1 In their procedure, autogenous marrow from the ilium was used.1 Although these authors demonstrated noteworthy success, significant morbidity was associated with such procedures, and clinicians sought alternative grafting materials that could achieve high levels of success without the need for secondary surgical sites or the associated complications. In 1996, the Academy of Osseointegration's Sinus Graft Consensus Conference concluded that several grafting materials were acceptable and that no material was superior to another.2 Since the first consensus conference, the introduction of additional novel graft materials and the emergence of long-term histomorphometric and clinical studies has influenced clinical practice and the graft materials used for lateral sinus augmentation.
Indications for Sinus Bone Grafting
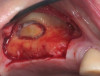
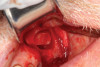
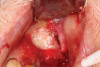
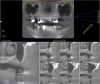
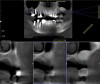
Dental implants are a predictable treatment option for the replacement of missing teeth. However, adjunctive surgical procedures may be required prior to implant placement. Sinus augmentation is indicated for cases in which deficient vertical bone height in the posterior region of the maxilla prevents dental implant placement. If inadequate residual bone height is present coronal to the maxillary sinus, the lateral sinus augmentation technique is recommended because it allows for the placement of larger volumes of graft material as well as greater access and visibility. In this technique, an osteotomy is made over the lateral sinus wall using rotary burs or a piezoelectric tip, taking care to keep the sinus mucosa intact (Figure 1). The sinus mucosa is then elevated (Figure 2), and a bone graft material is placed (Figure 3). Once healed, a significant increase in height and volume of bone can be seen radiographically (Figure 4 and Figure 5).
Bone Grafting Materials
In order to be considered ideal, a bone graft material should possess several properties, including the ability to provide adequate space maintenance over time; permit the diffusion of bone cells and nutrients throughout the graft; promote vascular ingrowth; promote bone cell attachment, migration, and proliferation; offer compressive strength, elasticity, and dimensional stability; and resorb during remodeling in an appropriate time frame.3 Although there is currently no available biomaterial that possesses all of these properties, understanding the characteristics of the available bone graft materials can help in making recommendations for the selection of optimal bone graft materials for lateral sinus augmentation in individual patient situations (Table 1).
Autografts
Autogenous bone grafts are taken from the same individual who will receive the graft. Autogenous bone is the only bone grafting material that may have osteogenic properties, which makes it the gold standard. A systematic review by Danesh-Sani and colleagues evaluated the histomorphometric results after sinus floor augmentation with different graft materials, and the use of autogenous bone resulted in the highest amount of new bone formation.4 Autogenous bone also has a shorter healing time when compared with bone substitutes. It has been suggested that sinuses grafted with autogenous bone may have healing times of 3 to 4 months when compared with the 8 to 10 months required for other bone substitutes.5,6 Other advantages of autogenous bone grafts include lower material costs and no risk of disease transmission or antigenicity. According to the results of one systematic review, at sites with 6.0 mm or less residual bone height, rough implants demonstrated a high survival rate (> 96%) in all graft materials.7 However, those placed in sinuses grafted with autogenous bone demonstrated a significantly higher 3-year survival rate (99.8%) when compared with those placed in sinuses grafted with other materials.7 It should be noted that the use of autogenous bone graft is associated with an increased risk of morbidity and potential surgical complications because a secondary donor site is required.1,2 The use of autogenous bone is an acceptable option for lateral sinus augmentation, but the advantages and disadvantages should be considered in case selection.
Allografts
Allografts are harvested from another individual of the same species. Demineralized freeze-dried bone allograft (DFDBA) and freeze-dried bone allograft (FDBA) are commonly used in dentistry. The utility of DFDBA is limited in sinus augmentation for several reasons, including the following8-10:
• It is not radiopaque, which may prevent the identification of unrecognized perforation radiographically.
• Its resorption rate is generally faster than other bone replacement materials, which may decrease the volumetric stability over time.
• Its inconsistent amount and availability of bone morphogenic proteins may make its osteoinductive properties clinically irrelevant for this application.
For these reasons, FDBA is the allograft material of choice for sinus augmentation. FDBA is radiopaque and offers an osteoconductive scaffold for bone ingrowth and maintenance. In addition, it has been demonstrated that allograft materials result in significantly greater vital bone formation when compared with xenograft materials at 26 to 32 weeks after sinus augmentation (28% versus 12%, respectively).11
Xenografts
Xenografts are taken from a donor of another species. In dentistry, xenograft material is typically derived from porcine, equine, or bovine sources. Implants placed at sites with previous sinus augmentation using xenograft materials have demonstrated favorable clinical outcomes.12 Xenograft bone substitutes have a slow resorption rate, which allows for the maintenance of graft height and volume. In addition, the high radiopacity of xenograft material facilitates ease of confirmation of graft localization and containment, which can help ensure that a large perforation was not unrecognized. When compared with using autogenous bone, xenograft bone substitutes reduce the incidence of morbidity, may decrease intrasurgical time, and can offer an essentially unlimited supply. However, it is important to consider that xenografts may require increased healing time due to their slow absorption rates and that, although implant success rates remain high at sites with xenograft materials, histomorphometric studies demonstrate that they result in a lower percentage of vital bone formation when compared with other materials.12,13
Alloplastic Grafts
Alloplastic bone grafts are made of synthetic materials. Alloplasts are osteoconductive, so they can provide a scaffold for new bone formation.14 Several studies have demonstrated the effectiveness of their use in sinus augmentation either alone or in combination with other bone graft materials.15,16 Patients may prefer to avoid using bone grafts from allograft or xenograft sources due to health, moral, religious or other concerns. Because alloplastic bone graft materials are synthetic, there is no risk of disease transmission and the antigenicity associated with them is low, which may increase patient acceptance. The amount of new bone formation that can be achieved varies based upon the individual qualities of the alloplast but is, in general, lower than that of the other graft materials available.
Stabilized Blood Clot
The use of a stabilized blood clot for lateral sinus augmentation is a recent concept that was first published by Lundgren and colleagues in 2004.17 They found that all of the implants remained clinically stable and that comparisons of preoperative and postoperative computed tomography (CT) scans showed new bone formation.17 Further studies have demonstrated radiographic bone formation and micro-CT/histological evidence of de novo bone formation with the use of venous coagulum for sinus augmentation.18 Long-term randomized control studies are needed to provide additional information about the comparative efficacy of stabilized blood clots as graft materials.
Emerging Adjunctive Graft Materials
When used in combination with a bone replacement material or carrier, several adjunctive materials have shown promise in their abilities to enhance healing, reduce postoperative complications, improve bone formation, and potentially shorten the time to implant placement. These bioactive materials, which are either patient derived or recombinant, may play a role in improving outcomes in lateral sinus augmentation.
Platelet Concentrates
The use of platelet concentrates is an enticing adjunct to implant dentistry. These autologous products are created by spinning the patient's own blood in a centrifuge. The two most commonly used platelet concentrates are platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). The rationale for using platelet concentrates is that they contain a high number of autologous growth factors.19
PRP has been shown to enhance the healing of bone and soft tissues.20 In sinus augmentation procedures, the adjunctive use of PRP does not demonstrate a significant improvement in hard-tissue healing or implant survival20; however, it does offer advantages. It has been reported that the use of PRP can improve patient quality of life and satisfaction postoperatively.21 Patients who received PRP experienced significantly less swelling, pain, and hematoma as well as improved functional activities.21
PRF has been shown to reduce tissue inflammation, promote the vascularization of bone tissue, accelerate new bone formation, and improve scaffold mechanics.22 PRF has become favored over PRP due to its stronger effect on osteoblasts and handling properties, especially when used as an adjunct to bone replacement grafting.22 It also has been reported to be easier to prepare and less expensive. Like PRP, PRF has not been shown to significantly improve vital bone formation or implant survival rates. In a meta-analysis by Liu and colleagues,23 they found no improvement in hard-tissue outcomes such as vital bone nor was there any improvement in implant survival rates. PRF has similar properties to PRP regarding its ability to improve immediate postoperative quality of life and can be considered advantageous to use in that regard.
rhBMP-2
Bone morphogenic proteins are growth factors that induce osteoblast differentiation.24 Recombinant human bone morphogenic protein-2 (rhBMP-2) is the most studied variant, and it has several applications in implant dentistry. A 6-month postoperative histological comparison of rhBMP-2 carried by an absorbable collagen sponge with autogenous grafts found no difference between the rhBMP-2 sites and the autogenous graft sites.25 However, rhBMP-2 is relatively expensive and may be associated with significant morbidity, including seroma formation and significant postoperative swelling.26
rhPDGF-BB
Platelet-derived growth factor-BB (PDGF-BB) is a cytokine that is released during normal wound healing. The recombinant human form, rhPDGF-BB, is approved for use in periodontal regeneration along with a beta-tricalcium phosphate (β-TCP) carrier, and it has also been used off-label as an adjunct for lateral sinus augmentation. Froum and colleagues27 compared the use of anorganic bovine bone matrix (ABBM) alone with ABBM plus rhPDGF-BB (without β-TCP granules). When they assessed the bone at 4 to 5 months, there was significantly more vital bone in the rhPDGF-BB group. At 7 to 9 months, no differences were noted between groups. These results, along with those of another study,28 suggest that the adjunctive use of ABBM with rhPDGF-BB may permit earlier implant placement, but additional research is needed to establish an optimal protocol.28
Stem Cells/Scaffolds
The use of stem cells in implant dentistry and medicine as a whole has the potential to improve outcomes. Stem cells have the ability to differentiate into specialized cell lines and can be harvested from a multitude of sources.29 Their use in sinus augmentation procedures is feasible; however, the number of studies performed thus far has been limited. Shayesteh and colleagues30 treated 6 patients with mesenchymal stem cells and a β-TCP/hydroxyapatite scaffold. Although implants placed at 3 months exhibited stability, this was not a controlled study. Stem cells may offer a promising way to expedite treatment time for patients and warrant further investigation. The current costs associated with stem cell and scaffold use is prohibitive to their use for many dental applications, but the development of cost-effective mechanisms to utilize stem cells may facilitate wider use of this technology.
Summary
Lateral sinus augmentation is a predictable way to increase vertical bone height and facilitate implant placement in the posterior region of the edentulous maxilla. Many bone grafting materials have evidence-based research supporting their use in sinus augmentation. Having a strong understanding of the characteristics of the available materials can guide the clinician in optimal graft selection. There is emerging evidence supporting the use of adjunctive bioactive products for sinus augmentation. These bioactive materials may provide opportunities to accelerate healing or minimize postoperative discomfort; however, additional randomized control trials are needed.
Queries regarding this course may be submitted to authorqueries@aegiscomm.com
About the Authors
Maggie Misch-Haring, DMD
Periodontal Resident
University of Alabama at Birmingham
School of Dentistry
Birmingham, Alabama
William Liska, DDS
Private Practice
Redding, California
Maria L. Geisinger, DDS, MS
Diplomate
American Board of Periodontology
Professor and Director
Advanced Education Program in Periodontology
University of Alabama at Birmingham School of Dentistry
Birmingham, Alabama
References
1. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38(8):613-616.
2. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants. 1998;13(Suppl):11-45.
3. Haugen HJ, Lyngstadaas SP, Rossi F, Perale G. Bone grafts: which is the ideal biomaterial? J Clin Periodontol. 2019;46(Suppl 21):92-102.
4. Danesh-Sani SA, Engebretson SP, Janal MN. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: a systematic review and meta-analysis. J Periodontal Res. 2017;52(3):301-312.
5. Froum SJ, Tarnow DP, Wallace SS, et al. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic and histomorphometric analysis--Part 2 of an ongoing prospective study. Int J Periodontics Restorative Dent. 1998;18(6):529-543.
6. Soardi CM, Spinato S, Zaffe D, Wang HL. Atrophic maxillary floor augmentation by mineralized human bone allograft in sinuses of different size: a histologic and histomorphometric analysis. Clin Oral Implants Res. 2011;22(5):560-566.
7. Pjetursson BE, Tan WC, Zwahlen M, Lang NP. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J Clin Periodontol. 2008;35(8 Suppl):216-240.
8. Borg TD, Mealey BL. Histologic healing following tooth extraction with ridge preservation using mineralized versus combined mineralized-demineralized freeze-dried bone allograft: a randomized controlled clinical trial. J Periodontol. 2015;86(3):348-355.
9. Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996;17(11):1127-1131.
10. Blum B, Moseley J, Miller L, et al. Measurement of bone morphogenetic proteins and other growth factors in demineralized bone matrix. Orthopedics. 2004;27(1 Suppl):S161-S165.
11. Froum SJ, Wallace SS, Elian N, et al. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006;26(6):543-551.
12. Wallace SS, Tarnow DP, Froum SJ, et al. Maxillary sinus elevation by lateral window approach: evolution of technology and technique. J Evid Based Dent Pract. 2012;12(3 Suppl):161-171.
13. Papageorgiou SN, Papageorgiou PN, Deschner J, et al. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J Dent. 2016;48:1-8.
14. Misch CE, Dietsh F. Bone grafting materials in implant dentistry. Implant Dentistry. 1993;2(3):158-167.
15. Browaeys H, Bouvry P, De Bruyn H. A literature review on biomaterials in sinus augmentation procedures. Clin Implant Dent Relat Res. 2007;9(3):166-177.
16. Wheeler SL. Sinus augmentation for dental implants: the use of alloplastic materials. J Oral Maxillofac Surg. 1997;55(11):1287-1293.
17. Lundgren S, Andersson S, Gualini F, Sennerby L. Bone reformation with sinus membrane elevation: A new surgical technique for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2004;6(3):165-173.
18. Beaudry KA, Geurs NC, Lemons JE, Reddy MS. Bone formation under the elevated sinus membrane using venous coagulum. Clin Adv in Per. 2013:3(4):200-207.
19. Agrawal AA. Evolution, current status, and advances in application of platelet concentrate in periodontics and implantology. World J Clin Cases. 2017;5(5):159-171.
20. Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant, and reconstructive surgery. Curr Pharm Biotechnol. 2012;13(7):1231-1256.
21. Del Fabbro M, Corbella S, Ceresoli V, et al. Plasma rich in growth factors improves patients' postoperative quality of life in maxillary sinus floor augmentation: preliminary results of a randomized clinical study. Clin Implant Dent Relat Res. 2015;17(4):708-716.
22. Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: a critical review. J Conserv Dent. 2013;16(4):284-293.
23. Liu R, Yan M, Chen S, et al. Effectiveness of platelet-rich fibrin as an adjunctive material to bone graft in maxillary sinus augmentation: a meta-analysis of randomized controlled trials. Biomed Res Int. 2019:7267062. doi: 10.1155/2019/7267062.
24. Diaz-Sanchez RM, Yanex-Vico RM, Olavarria AF, et al. Current approaches of bone morphogenic proteins in dentistry. J Oral Implantol. 2015;41(3):337-342.
25. Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009:67(9):1947-1960.
26. James AW, LaChaud G, Shen J, et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016;22(4):284-297.
27. Froum SJ, Wallace S, Cho SC, et al. A histomorphometric comparison of Bio-Oss alone versus Bio-Oss and platelet-derived growth factor for sinus augmentation: a postsurgical assessment. Int J Periodontics Restorative Dent. 2013;33(3):269-279.
28. Kubota A, Sarmiento H, Alqahtani MS, et al. The use of recombinant human platelet-derived growth factor for maxillary sinus augmentation. Int J Periodontics Restorative Dent. 2017;37(2):219-225.
29. Zheng C, Chen J, Liu S, Jin Y. Stem cell-based bone and dental regeneration: a view of microenvironment modulation. Int J Oral Sci. 2019;11(3). doi: 10.1038/s41368-019-0060-3.
30. Shayesteh YS, Khojasteh A, Soleimani M, et al. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(2):203-209.