You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
In the 1950s, an orthopedic surgeon named Per-Ingvar Brånemark was researching the anatomy of blood flow utilizing an optical device that was placed into the femur of a rabbit to permit observation of the animal's osseous microcirculation. The device was encased in a titanium chamber, and when he went to remove it from the animal, he found that the metal chamber and the surrounding bone had become inseparable from each other. He referred to this phenomenon of the bone "fusing" with the nonbiological material as "osseointegration," and his research shifted to study it in-depth.1 Prior to this discovery, various metals that had been routinely utilized in orthopedic implants had been used in the fabrication of dental implants, including tantalum, cobalt-chromium, and stainless steel. However, those metals had no reported evidence of the osseointegration effect that Brånemark was the first to report was associated with titanium-based metals.2 Brånemark's studies led to the placement of titanium dental implants in dogs in the early 1960s, and in 1965, he placed the first titanium implants in a human volunteer. Those results, as well as his experience with other patients over a 10-year period, were published in 1977.3 Titanium is a dense hard metal; therefore, commercially pure titanium (CP-Ti) was initially selected for milling because this version of the metal was softer and also because it was identical to the titanium used in the chambers that Brånemark had designed when he first stumbled onto its ability to osseointegrate with bone. Those early titanium implants were fabricated from CP-Ti in grade 1 and grade 2.
Titanium is a highly reactive element. Because it is very reactive with oxygen, it must be melted in a vacuum or under inert gas in order to prevent oxidation and the incorporation of oxygen, which can lead to an increase in brittleness with a loss in strength. This same reactivity with oxygen is also responsible for many of titanium's favorable properties. When a titanium implant reacts with oxygen, it forms an oxide layer on the surface that is beneficial and aids in osseointegration with the adjacent bone. Titanium oxidizes almost instantaneously in air to form a tenacious but stable oxide layer approximately 10 nm thick.5 This oxide layer also provides a highly biocompatible and corrosion-resistant surface. All of this makes the casting of titanium difficult, so implants are fabricated by milling bar stock of the titanium type selected.4
Mechanical Properties of Titanium by Grade
CP-Ti is available in four grades, numbered 1 to 4, based on the purity and the content of the oxygen used to process it.6 These grades differ in their corrosion resistance, ductility, and strength and are defined by their oxygen and iron content, which have a substantial effect on the mechanical and physical properties of the metal. As titanium's concentration of oxygen or iron increases, its mechanical strength increases while its ductility decreases. An increase in the grade number of CP-Ti equates with an increase in the amount of impurities. Therefore, grade 1 is the softest and most ductile type of CP-Ti, whereas grade 4 is significantly stronger and less malleable than the lower grades.7 There are some disadvantages to CP-Ti. Grades 1 through 4 have relatively low mechanical strength, a high Young's modulus (ie, elastic modulus), and poor wear resistance, which may lead to implant fracture when mechanical overloading occurs.8 Of the CP-Ti grades, grade 4 has the highest tensile strength and yield strength, so dentistry moved to this version for dental implants in the early 1990s.
The mechanical properties of titanium, such as its strength, creep resistance, and fracture resistance, can be improved by alloying it with a wide range of elements (eg, aluminum, vanadium, tantalum, zirconium).9 To overcome some weakness issues reported with CP-Ti, including grade 4, a titanium alloy referred to as Ti-6Al-4V grade 5 was introduced later in the 1990s. Ti-6Al-4V is a titanium alloy that contains 6% aluminum and 4% tantalum, and it is the most common alloy used in dental implants. Titanium alloys exist in three structural forms: alpha, beta, and alpha-beta. The alpha form of titanium alloy has a closely packed hexagonal crystallographic structure, whereas the beta alloys have a body-centered cubic form. These different phases originate when pure titanium is mixed with other elements, such as aluminum and vanadium, in certain concentrations and then cooled. Aluminum is an alpha-phase stabilizer, which increases the strength of the alloy while it decreases its density. Alternatively, vanadium is a beta-phase stabilizer.10
With the addition of aluminum or vanadium to titanium, the alpha-to-beta transformation changes over a range of temperatures. Depending on the composition and heat treatment, both the alpha and beta forms may coexist.11 The alpha-beta titanium combination alloy is the most commonly used for the fabrication of dental implants. Heat treatment of the alloy generates a fine precipitation, improving its strength and resulting in more favorable mechanical and physical properties, including a relatively low density and a high resistance to fatigue and corrosion.12
The disadvantages of using CP-Ti grades 1 through 4 for dental implants include relatively low mechanical strength, poor wear resistance, and difficulty in improving the mechanical properties without reducing biocompatibility. When higher strength is desired, the alloyed titanium is produced with extra-low interstitial (ELI) solutes, such as small amounts of oxygen, carbon, nitrogen, and hydrogen.9
Several properties are important to consider in a comparison of the different grades of commercially pure titanium and titanium alloy, including elastic modulus, tensile strength, yield strength, and elongation (see Table). Elastic modulus refers to the ability of a material to bend before fracture occurs. The higher the value, the more the metal can bend before failure results. The goal is for the elastic modulus of the material to approximate the elastic modulus of the surrounding bone so that when a load is placed on the implant and transferred to the surrounding bone, both media (ie, bone and implant) are within a similar range. It has been reported that with an increase in implant diameter, the stress and strain on the implant-bone interface significantly decrease.13 This is also affected by the length of the implant.14 In addition, increasing the diameter and length of the implant has been reported to decrease the stress and strain on the alveolar crest, and this should be considered no matter what material the implant is fabricated from.15 Although Ti-6Al-4V grade 5 alloy has a higher elastic modulus, it does not significantly differ from that of the CP-Ti grades. Therefore, it is not as important a factor in differentiating these different metals.7,16
Tensile strength, which is measured in megapascals (MPa), is an indicator of the "ultimate" strength of a material before failure occurs. Regarding dental implants, tensile strength refers to how high of a load can be placed on an implant before failure of the material results, which typically involves a fracture occurring in the implant.17,18 Although the tensile strength of CP-Ti grades can be improved by a cold-work process, this procedure makes the material more brittle.19,20 Dental implants undergo repeated (ie, cyclic) loading and are not under a constant load during normal function. Due to the complexity of the loading associated with dental implants, the potential for brittle failure of higher grades of CP-Ti, including grade 4, should be considered when selecting from implants fabricated from different metals.
Yield strength, which is also measured in MPa, is the fatigue strength of a material. Materials used for dental implants should have high yield strength to prevent brittle fracture under cyclic loading. Similar to tensile strength, the metal needs to possess high yield strength in order to be able to resist fracture and avoid catastrophic failure of the implant. Titanium alloy grade 5 has a yield strength that is almost double that of CP-Ti grade 4, which itself demonstrates increased strength when compared with the other grades of CP-Ti. This increase in value permits greater cyclic loads to be placed on the implant before a fracture results, either at the connection at the coronal aspect of the implant or in the body of the implant in cases where bone loss places greater lengths of the implant supracrestally.21
When subjected to loading, cyclic or static, metals may undergo "creep." Creep is a type of metal deformation that occurs at stresses below the yield strength of a metal.22 Regarding dental implants, this may lead to alteration of the platform of the implant through distortion of the connection or by the coronal aspect at the platform becoming "out of round." With an internal type of connection, under continued loading, this may eventually lead to loosening of the abutment on the implant as the intimacy of the fit between the parts decreases or even a fracture of the implant coronally. An external hex connection undergoing creep will demonstrate rounding of the points of the hex. The potential for creep is determined by measuring the percentage of elongation of a material. A higher percentage of elongation indicates a softer metal and higher possible degree of creep. CP-Ti demonstrates a decreasing percentage of elongation from grade 1 to 4 due to a decrease in the softness of the metal. Furthermore, comparisons of CP-Ti grade 4 with Ti-6Al-4V grade 5 reveal a significantly lower potential for creep associated with the alloy.20,23
Mechanical Problems and Metal Composition
The occlusal contact of implant-retained prosthetics with the opposing dentition results in loading of the implants and the surrounding bone supporting them. There are multiple factors that can result in mechanical overloading, including occlusal high spots, off-axis contact of the occluding surfaces, and parafunctional habits (eg, clenching and grinding).24-26 Over time, the cyclical process of mechanical loading can lead to structural failure of an implant if the metal that it is fabricated from has physical properties that are insufficient to manage that loading.27 This may subsequently lead to fracture of the implant or deformation of the crestal portion at the implant's connection.
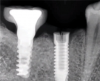
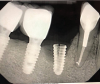
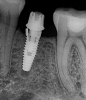
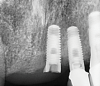
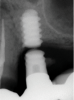
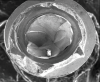
Implants with internal hex connections are more prone to failure at the connection than those with external hex connections.28 This is related to the thickness of the metal at the thinnest point between the internal surface of the interface and the external surface at the crestal area of the implant. When overloaded, implants with an internal hex connection may present with fractures at the points of the hex.29 These points are where the metal is the thinnest at the crestal portion of the implant and where stress is concentrated during overloading. This is less problematic in wider diameter implants because the metal is thicker in this area of the implant; however, in standard or narrow diameter implants, fracture may result, causing catastrophic failure of the implant (Figure 1). This may also occur in other internal implant connection types, such as trilobe connections, especially when the crestal thickness of the implant is minimal, leading to fracture of the coronal aspect of the implant (Figure 2). Implants with conical connections are not immune to potential fracture in this area, and the thin walls of some standard or narrow diameter models may split even without the isolated stress points observed in internal hex-, trilobe-, and octagon-type connections (Figure 3).30 Sometimes, fractures of implants at the coronal aspect are not clinically visible but, instead, are identified radiographically either after a patient complaint of pain in the area, due to the presence of soft-tissue inflammation, or during a routine examination with no patient complaint (Figure 4).
Coronal implant fracture, whether it is incomplete (Figure 5) or involves a visible loss of a portion of the implant platform (Figure 6 and Figure 7), results in catastrophic failure of the implant. The presence of any fracture in the coronal aspect of an implant necessitates explantation. These implants cannot be salvaged because an incomplete fracture will continue to propagate due to micromovement of the abutment and implant at the connection during function. Off-axis loads are amplified when there is a longer distance between the implant's platform and the most coronal surface of the restoration because the fulcrum arm is increased, allowing higher loading forces at the platform.31
In addition to fractures at the coronal aspect, fractures may also occur along the length of the body when sufficient overload has been placed on an implant that is well integrated but has undergone crestal bone loss.31 Because bone loss progresses in an apical direction, when off-axis loads are applied, the fulcrum arm on the implant increases, leading to greater loads at the crest. This can result in fracture of the body of the implant at that location (Figure 8). Implants fabricated from CP-Ti grade 4 in standard or narrow diameters may be more prone to these potential catastrophic failures than those same implant designs in wider diameters; therefore, the metal that the implant is fabricated from should be considered during selection.
Implants with external hex connections are not as prone to fracture at the interface as implants with internal connections.28 This is because the metal is thicker at the coronal aspect of the implant between the threaded channel for the abutment and the implant's outer diameter on implants with an external hex connection. Despite this, exceeding the mechanical loading limits may be more likely to result in implant failure if the implant is fabricated from a CP-Ti grade 4 metal than if it is fabricated from a titanium alloy.
As discussed, yield strength defines the stress at which metal begins to plastically deform. When presented with off-axis loads over time, an external hex connection on an implant may undergo creep, which leads to rounding of the points because this is where the stress is concentrated. As the points on the external hex deform, they permit greater micromovement of the abutment, which leads to greater stress being placed on the abutment screw and can result in fracture of the screw (Figure 9). When an implant is fabricated from CP-Ti, even grade 4, there is a greater potential for this problem than if it is fabricated from titanium alloy grade 5; however, it occurs less frequently in wider diameter implants.
Conclusion
Today, dental implants are offered in both CP-Ti grade 4 and titanium alloy grade 5, depending on the specific manufacturer and design. With regard to osseointegration, no clinically significant differences have been reported between these two metal types. Implant selection should be based on the properties of the metal used in fabrication and its resistance to potential fracture in situations when mechanical overloading may occur during function. Unfortunately, when planning a case, it is frequently difficult to predict if a patient will exceed loading limits, and oftentimes, this isn't known until the implant is placed and a failure has actually occurred. Titanium alloy grade 5 provides greater strength than CP-Ti grade 4, and the selection of implants fabricated from this metal over those implants made of CP-Ti should be considered a clinical improvement. This choice can help to eliminate the potential for failures related to the metal that implants are fabricated from.
Queries regarding this course may be submitted to authorqueries@aegiscomm.com
About the Author
Gregori M. Kurtzman, DDS
Master
Academy of General Dentistry
Diplomate
International Congress of Oral Implantologists
Private Practice
Silver Spring, Maryland
References
1. Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50(3):399-410.
2. Saini M, Singh Y, Arora P, et al. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015;3(1):52-57.
3. Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1-132.
4. Philip GB, Jhamb M, George E, Jhamb R. Titanium and its role in dentistry. International Journal of Scientific and Research Publications. 2017;7(5):602-608.
5. Wang RR, Fenton A. Titanium for prosthodontic applications: a review of the literature. Quintessence Int. 1996;27(6):401-408.
6. Liu X, Chen S, Tsoi JKH, Matinlinna JP. Binary titanium alloys as dental implant materials-a review. Regen Biomater. 2017;4(5):315-323.
7. Masa R, Braunitzer G. Titanium and its alloys in dental implantology. Implants. 2017;4:6-10.
8. Nicholson JW. Titanium alloys for dental implants: a review. Prosthesis. 2020;2(2):100-116.
9. Elias CN, Fernandes DJ, Resende CR, Roestel J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. Dent Mater. 2015;31(2):e1-e13.
10. González JEG. Mirza-Rosca, JC. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal Chem. 1999;471(2):109-115.
11. McCracken M. Dental implant materials: commercially pure titanium and titanium alloys. J Prosthodont. 1999;8(1):40-43.
12. Osman RB, Swain MV. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials (Basel). 2015;8(3):932-958.
13. Ding X, Zhu XH, Liao SH, et al. Implant-bone interface stress distribution in immediately loaded implants of different diameters: a three-dimensional finite element analysis. J Prosthodont. 2009;18(5):393-402.
14. Robau-Porrua A, Pérez-Rodríguez Y, Soris-Rodríguez LM, et al. The effect of diameter, length and elastic modulus of a dental implant on stress and strain levels in peri-implant bone: a 3D finite element analysis. Biomed Mater Eng. 2020;30(5-6):541-558.
15. Ding X, Liao SH, Zhu XH, et al. Effect of diameter and length on stress distribution of the alveolar crest around immediate loading implants. Clin Implant Dent Relat Res. 2009;11(4):279-287.
16. Patel NR, Gohil PP. A review on biomaterials: scope, applications & human anatomy significance. Int J Emerg Technol Adv Eng. 2012;2(4):91-101.
17. ASTM F136 - 13 Standard specification for wrought titanium-6aluminum-4vanadium ELI (extra low interstitial) alloy for surgical implant applications (UNS R56401). ASTM International website. http://www.astm.org/cgi-bin/resolver.cgi?F136. Accessed April 20, 2021.
18. ASTM F67 - 13(2017) Standard specification for unalloyed titanium, for surgical implant applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700). ASTM International website. http://www.astm.org/cgi-bin/resolver.cgi?F67. Accessed April 20, 2021.
19. Okazaki Y. Comparison of fatigue properties and fatigue crack growth rates of various implantable metals. Materials (Basel). 2012;5(12):2981-3005.
20. Muddugangadhar BC, Amarnath GS, Tripathi S, Divya SD. Biomaterials for dental implants: an overview. Int J Oral Implantology Clin Res. 2011;2(1):13-24.
21. Saini M, Singh Y, Arora P, et al. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015;3(1):52-57.
22. Khraisat A. Two implant/abutment joint designs: a comparative finite element analysis. Int J Oral Maxillofac Implants. 2013;28(2):e83-e87.
23. Sykaras N, Iacopino AM, Marker VA, et al. Implant materials, designs, and surface topographies: their effect on osseointegration. a literature review. Int J Oral Maxillofac Implants. 2000;15(5):675-690.
24. Piattelli A, Piattelli M, Scarano A, Montesani L. Light and scanning electron microscopic report of four fractured implants. Int J Oral Maxillofac Implants. 1998;13(4):561-564.
25. Lobbezoo F, Brouwers JE, Cune MS, Naeije M. Dental implants in patients with bruxing habits. J Oral Rehabil. 2006;33(2):152-159.
26. Lee DW, Lee DW, Park KH, Moon IS. The effects of off-axial loading on periimplant marginal bone loss in a single implant. J Prosthet Dent. 2014;112(3):501-507.
27. Dănilă V, Augustin M. [Occlusal overload--a risk factor in implant based prostheses]. Rev Med Chir Soc Med Nat Iasi. 2010;114(1):214-217.
28. Yi Y, Koak JY, Kim SK, et al. Comparison of implant component fractures in external and internal type: a 12-year retrospective study. J Adv Prosthodont. 2018;10(2):155-162.
29. Yu HC, Kim YK. Fractures of implant fixtures: a retroscccpective clinical study. Maxillofac Plast Reconstr Surg. 2020;42(1):13.
30. Jacobs N, Seghi R, Johnston WM, Yilmaz B. Displacement and performance of abutments in narrow-diameter implants with different internal connections. J Prosthet Dent. 2021;S0022-3913(20)30709-5. doi: 10.1016/j.prosdent.2020.11.008.
31. Gehrke SA, Dedavid BA, Prados-Frutos JC. Effects of different switched or not-switched implant and abutment platform designs and marginal bone loss on fracture strength: an in vitro study. J Prosthet Dent. 2021;S0022-3913(20)30767-8. doi: 10.1016/j.prosdent.2020.11.038.