You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
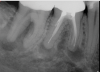
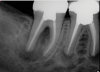
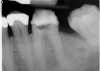
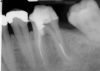
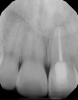
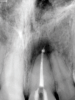
In endodontics, the techniques and materials are constantly evolving to achieve superior clinical outcomes and improve patient satisfaction. Bioceramic materials, which have historically been used in medicine primarily because of their biocompatibility with human tissue, are ceramic compounds that include alumina, zirconia, bioactive glasses, glass ceramics, hydroxyapatite, calcium silicates, and calcium phosphates.1 These bioactive materials have been favored over other dental materials for a variety of reasons, including their biocompatibility with human hydroxyapatite, enhanced ability to seal, antibacterial and antifungal effects, intrinsic osteoconductive activity, ability to chemically bond to tooth structure, and superior radiopacity (Figure 1 and Figure 2).2
Bioceramic Material Development
The first bioceramic material approved for endodontic use in the United States was the calcium silicate cement mineral trioxide aggregate (MTA).2,3 Beyond the general advantages associated with bioceramic materials, which include biocompatibility and osteoconductivity, MTA provides an effective restorative seal that forms calcium hydroxide upon setting and releases calcium ions for cell attachment and proliferation.1,2 In addition, MTA modulates cytokine production and creates an antibacterial microenvironment through its alkaline pH.1,2 Major shortcomings of MTA include its long setting time, high cost, difficult handling and manipulation, and propensity to discolor teeth. These shortcomings prevent the use of MTA in certain situations but are also indicative of important opportunities for improvement.3
Since the discovery of MTA, many alternative calcium and silicate-based bioceramic materials have been developed for use in endodontics (eg, Biodentine®, Septodont; EndoSequence BC Root Repair Material, Brasseler; TheraCal LC®, BISCO Inc.). These new materials were designed to address the shortcomings of MTA and improve upon the properties of bioceramics to optimize their clinical use.3 According to the manufacturers of these novel calcium silicate-based materials, their bioactive properties offer various advantages when compared with MTA, including the ability to penetrate through open dentinal tubules and interlock with human dentin to improve mechanical properties, lower cost, faster setting times, reduced bacterial contamination, lower porosity and levels of microleakage, and the ability to form a superior hermetic seal. Shortcomings of calcium silicate-based materials can include lower wash out resistance and poor radiopacity.2 Many of these products demonstrate similar antimicrobial and regenerative potential, and they result in lower levels of discoloration when compared with the use of MTA.3-5 Numerous variations of these calcium silicate products with nuanced differences in their properties already exist and are being actively developed. Although they exhibit similar levels of biocompatibility and alkalinity, patterns of cytokine expression, and regenerative potential,2 further research will shed greater light on the biomechanical differences among these materials and undoubtedly lead to the development of materials with even more favorable properties.2
Endodontic Applications
Bioceramic materials have many applications in endodontic procedures. These materials are widely used for vital pulp therapy and apexogenesis, including in pulp capping and pulpotomy procedures. In addition, bioceramics are used for apexification and regenerative endodontic procedures, perforation repairs, and as root end filling materials for apical surgery.2,3
Vital pulp therapy, which aims to preserve and maintain pulp tissue that has been compromised but not destroyed by extensive dental caries, dental trauma, or restorative procedures, has historically been utilized in immature permanent teeth to promote root maturation and improve their longevity. 6,7 However, new research supports its utility in mature permanent teeth as well.3,6,7Conservative management using vital pulp therapy not only provides a cost-effective solution but also eliminates many of the downsides to nonsurgical root canal therapy, including an increased lifetime risk of fracture and greater restorative needs.3,6,7 Vital pulp therapy procedures in permanent teeth include indirect pulp capping, direct pulp capping, partial pulpotomy, and complete pulpotomy.3 The overall success rate of vital pulp therapy has been reported to be between 73% and 99%.6 By procedure, the success rate of vital pulp therapy has been reported to be 100% for indirect pulp capping, 95% for direct pulp capping, 91% for partial pulpotomy, and 95% for complete pulpotomy.8
Bioceramic materials have become the standard for vital pulp therapy based on the strong research supporting their comparatively greater success. Formaldehyde-based medicaments, such as formocresol, which were historically used for vital pulp therapy, should no longer be used because these materials have been determined to be genotoxic and carcinogenic.9 Although calcium hydroxide has also been historically favored for use in vital pulp therapy procedures, it exhibits several unfavorable properties, including inability to bond to dentin, inadequate sealing and susceptibility to leakage, and dissolution in the moist environment of the oral cavity.3 Bioceramic materials do not share these deficiencies and are able to create a superior bond and seal.
Indirect Pulp Capping
Indirect pulp capping is recommended for both primary and permanent teeth that have developed carious lesions that encroach upon the pulp but do not exhibit signs or symptoms of pulpal inflammation or degeneration.3,7 In this procedure, a near complete excavation of caries is performed, leaving the deepest layer of leathery brown, but not soft, dentin behind onto which the material of choice is placed.7 In some versions of the procedure, after a period of a few months, the restorative material is removed along with any remaining stained dentin based on the presumption that tertiary dentin will form a protective layer for the pulp beneath.7 Alternatively, the remaining caries are left in place and presumed to remineralize beneath the indirect pulp cap.7
Bioceramic materials are ideal for indirect pulp capping due to their excellent sealing abilities. In fact, randomized controlled clinical trials have shown clinical, radiographic, and histologic success via newly formed dentin when bioceramics were used for indirect pulp capping in permanent teeth.10
Direct Pulp Capping
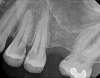
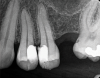
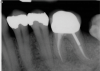
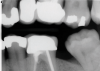
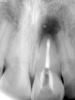
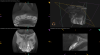
The goal of direct pulp capping is to cover healthy or reversibly inflamed pulps that have been mechanically exposed during operative procedures or trauma.3 In direct pulp capping procedures, the chosen material is applied directly to the pulp in an effort to maintain pulp vitality and encourage the formation of directly adjacent tertiary reparative dentin (Figure 3 and Figure 4).7 Clinical studies have shown a greater rate of success when direct pulp capping is performed using MTA (85%) when compared with calcium hydroxide (52%) and similar rates of success when MTA is compared with newer calcium silicate materials.5,11 Histologic studies have shown an increase in dentin bridge formation and lower levels of pulpal inflammation adjacent to bioceramic direct pulp caps, including those placed with MTA and those placed with newer bioceramic materials.3,12
Pulpotomy
The pulpotomy procedure involves the removal of diseased coronal pulp while preserving the vital radicular pulp.13 In immature teeth, this allows for continued root maturation with apical closure (ie, apexogenesis). Partial pulpotomy involves incomplete removal of the pulp tissue within the pulp chamber, whereas complete pulpotomy involves complete removal of the pulp tissue to the level of the canal orifices. The decision to perform a partial or complete pulpotomy is based on a provider's subjective assessment of the extent of inflammation and, generally, by his or her ability to achieve hemostasis in the remaining pulp tissue. Pulpotomies are germane to the management of both primary teeth and immature permanent teeth. The utility of pulpotomy as an alternative to nonsurgical root canal therapy in mature permanent teeth has traditionally been for emergency pain relief only; however, newer data involving the use of bioceramics suggest that pulpotomy could possibly be applied as a long-term alternative.13-15
Complete pulpotomies performed with MTA have reported success rates that range between 93% and 97%,15 and randomized clinical trials have shown that the clinical and histologic outcomes of pulpotomies performed with MTA and other bioceramics are similar.3 Although bioceramic materials have demonstrated success in pulpotomies, the evidence regarding their clinical and radiographic superiority over calcium hydroxide is, as of yet, inconclusive.3
Perforation Repair
Bioceramic materials, including MTA, offer a predictable means to repair iatrogenic furcal and lateral root perforations (Figure 5 through Figure 8).16 Overall, studies favor the use of MTA over other nonbioceramic materials for perforation repair,17,18 which is attributable to its superior seal. In addition, its inherent biocompatibility and osteoconductivity facilitates the repair of localized hard tissue damage. The key to successful perforation repair, regardless of the material utilized, is in the immediacy of its performance and completion before bacterial cross-contamination and inflammation-mediated bone loss can occur.17
Apical Surgery
Apical surgery, which is used to address recurrent or persistent endodontic pathoses, involves root-end resection followed by the placement of a root-end or retrograde filling material (Figure 9 through Figure 13).18,19 Clinical comparative studies have clearly established the greater predictability of surgical procedures that utilize bioceramics when compared with those that utilize alternative materials, such as amalgam or zinc oxide eugenol cements.18,19 As a result, bioceramics are considered the gold standard root-end filling materials.
Conclusion
Bioceramic materials have revolutionized many areas of endodontic clinical practice. Their inherent biocompatibility, antimicrobial effects, and excellent sealing properties make them ideal for procedures such as vital pulp therapy, perforation repair, and apical surgery. High-quality data demonstrate both clinical and histologic improvements in outcomes when bioceramics are compared with the materials previously utilized for these procedures. Since the introduction of MTA, newer iterations of bioceramic materials have led to improvements in setting time, handling properties, and staining potential. Further research will, no doubt, offer greater insight into additional novel applications for bioceramics, and the introduction of newer, even more ideal iterations of these materials is on the horizon.
Queries regarding this course may be submitted to authorqueries@aegiscomm.com
About the Authors
Ruchika Agrawal
DMD Candidate
Harvard School of Dental Medicine
Boston, Massachusetts
Brooke Blicher, DMD
Upper Valley Endodontics
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Clinical Instructor
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Rebekah Lucier Pryles, DMD
Upper Valley Endodontics
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Clinical Instructor
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Jarshen Lin, DDS
Director of Predoctoral Endodontics
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Clinical Associate
Department of Surgery
Massachusetts General Hospital
Boston, Massachusetts
References
1. Al-Haddad A, Che Ab Aziz ZA. Bioceramic-based root canal sealers: a review. Int J Biomater.2016;2016:9753210.
2. Kaur M, Singh H, Dhillon JS, et al. MTA versus Biodentine: review of literature with a comparative analysis. J Clin Diagn Res. 2017;11(8):ZG01-ZG05.
3. Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part I: vital pulp therapy. Int Endod J. 2018;51(2):177-205.
4. Lovato KF, Sedgley CM. Antibacterial activity of endosequence root repair material and proroot MTA against clinical isolates of Enterococcus faecalis. J Endod. 2011;37(11):1542-1546.
5. Machado J, Johnson JD, Paranjpe A. The effects of Endosequence Root Repair Material on differentiation of dental pulp cells. J Endod. 2016;42(1):101-105.
6. Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod. 2011;37(5):581-587.
7. Duncan HF, Galler KM, Tomson PL, et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J. 2019;52(7):923-934.
8. Asgary S, Hassanizadeh R, Torabzadeh H, Eghbal MJ. Treatment outcomes of 4 vital pulp therapies in mature molars. J Endod. 2018;44(4):529-535.
9. American Association of Endodontists. Concerning paraformaldehyde-containing endodontic filling materials and sealers: AAE position statement. American Association of Endodontists website. https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/paraformaldehydefillingmaterials.pdf. Revised April 2013. Reaffirmed October 2017.
10. Leye Benoist F, Gaye Ndiaye F, Kane AW, et al. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal®) in the formation of a dentine bridge: a randomised controlled trial. Int Dent J. 2012;62(1):33-39.
11. Kundzina R, Stangvaltaite L, Eriksen HM, Kerosuo E. Capping carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J. 2017;50(10):924-932.
12. Li Z, Cao L, Fan M, Xu Q. Direct pulp capping with calcium hydroxide or mineral trioxide aggregate: a meta-analysis. J Endod. 2015;41(9):1412-1417.
13. Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod.1978;4(8):232-237.
14. Hasselgren G, Reit C. Emergency pulpotomy: pain relieving effect with and without the use of sedative dressings. J Endod. 1989;15(6):254-256.
15. Taha NA, Ahmad MB, Ghanim A. Assessment of mineral trioxide aggregate pulpotomy in mature permanent teeth with carious exposures. Int Endod J. 2017;50(2):117-125.
16. Ford TR, Torabinejad M, McKendry DJ, et al. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):756-763.
17. Siew K, Lee AH, Cheung GS. Treatment outcome of repaired root perforation: a systematic review and meta-analysis. J Endod. 2015;41(11):1795-1804.
18. Torabinejad M, Parirokh M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J. 2018;51(3):284-317.
19. Kim S, Kratchman S. Microsurgery in Endodontics.1st ed. John Wiley & Sons, Inc.; 2018.